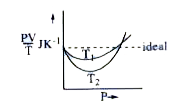A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
KINETIC THEORY OF GASES
AAKASH SERIES|Exercise EXERCISE-3(Various speed of gas molecules)|10 VideosKINETIC THEORY OF GASES
AAKASH SERIES|Exercise Problems|12 VideosKINETIC THEORY OF GASES
AAKASH SERIES|Exercise EXERCISE-2(Kinetic Theory of an ideal gas)|25 VideosKINEMATICS
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (PRACTICE SHEET (ADVANCED)) (Integer Type Questions)|12 VideosLAWS OF MOTION
AAKASH SERIES|Exercise PRACTICE EXERCISE|108 Videos
AAKASH SERIES-KINETIC THEORY OF GASES-EXERCISE-3(Kinetic Theory of an ideal gas)
- Given is the graph between PV/T and P for 1 gm of oxygen gas at two di...
Text Solution
|
- Three closed vessels A, B and C are at the same temperature T and cont...
Text Solution
|
- The mass of H(2) molecule is 3.32xx10^(-24) g .If 10^(23) hydrogen mol...
Text Solution
|
- The atomic mass of carbon (diamond) is 12.01 U and density is 2.22xx10...
Text Solution
|