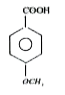
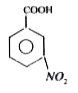
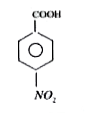
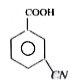
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise LECTURE SHEET - EXERCISE-III (Linked Comprehension Type Questions)|6 VideosELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise LECTURE SHEET - EXERCISE-III (Matrix Matching Type Questions)|1 VideosELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise LECTURE SHEET - EXERCISE-III (Straight Objective Type Questions)|29 VideosELECTRON MIGRATION EFFECTS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|10 VideosELEMENTS OF BORON FAMILY
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3|7 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ELECTRONIC EFFECTS AND REACTION INTERMEDIATES-LECTURE SHEET - EXERCISE-III (More than correct answer Type Questions)
- The following are more acidic than
Text Solution
|
- Write chemical equations for the following conversions : C(6)H(5)-CH...
Text Solution
|
- Which of the following is the strongest acid
Text Solution
|
- Which of the following statements is true
Text Solution
|
- correct acidity order of x, y and z is
Text Solution
|
- Rank the order of pKa values for each of the compound below
Text Solution
|