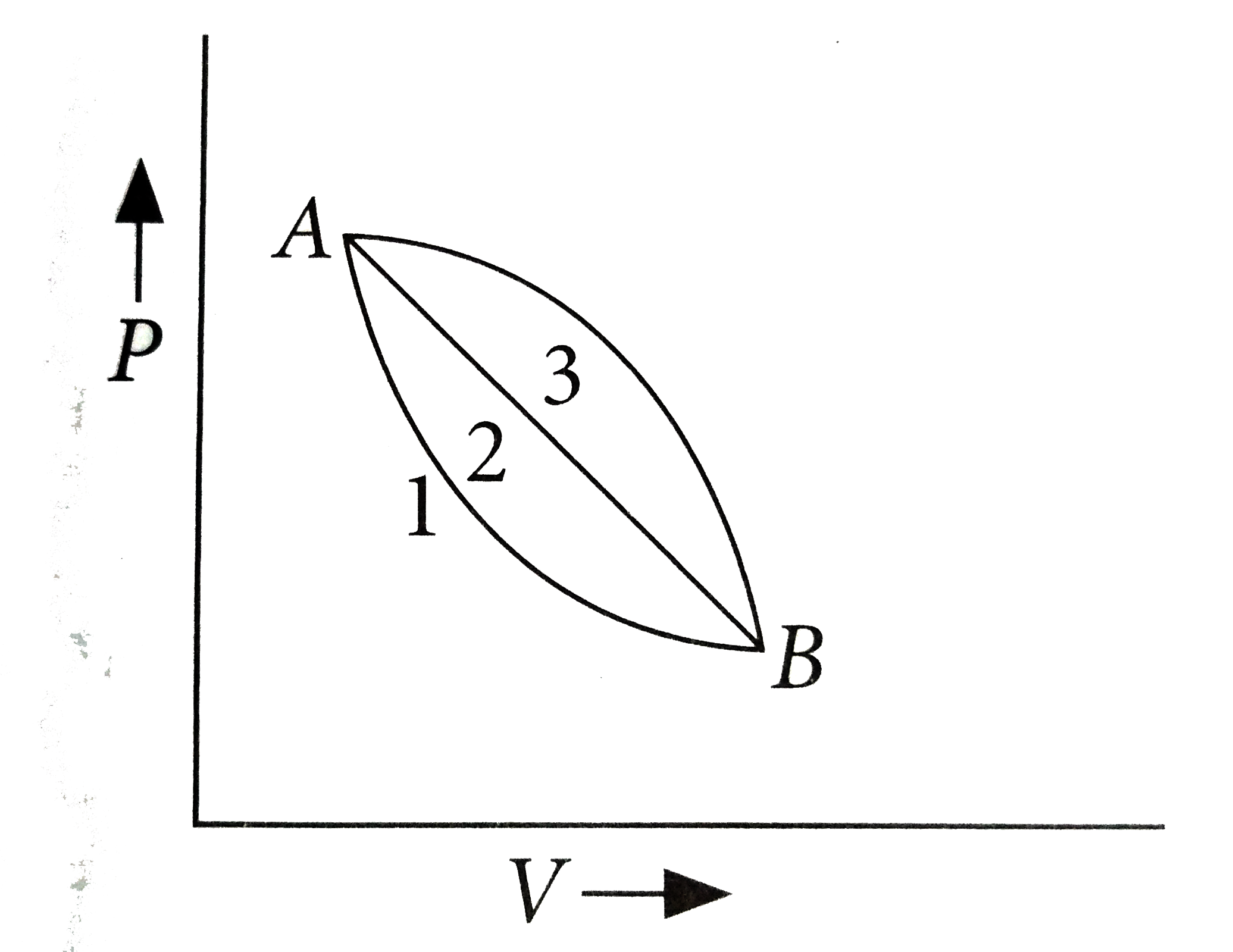
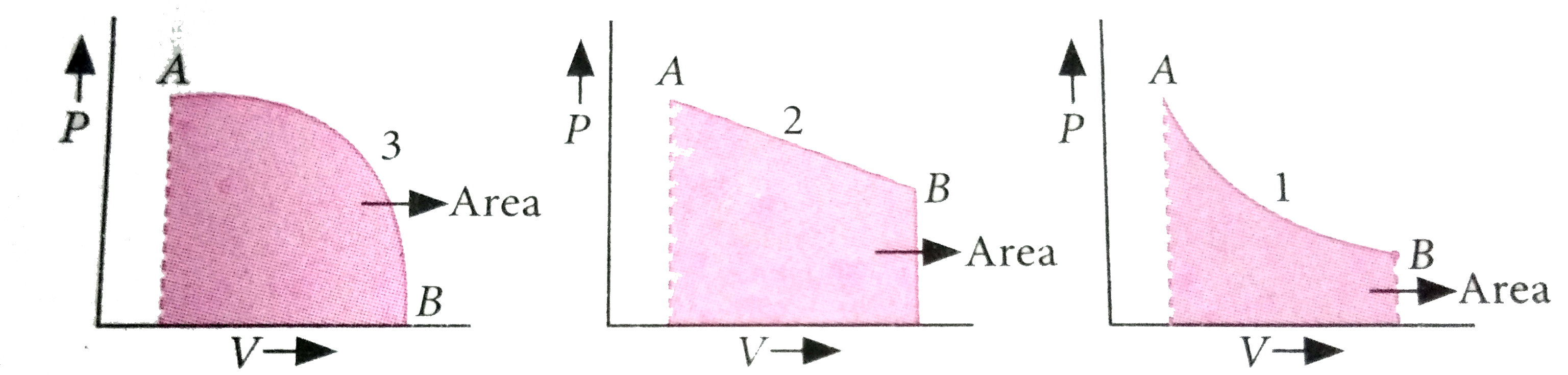
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
NCERT FINGERTIPS ENGLISH|Exercise NCERT EXEMPLAR PROBLEMS|11 VideosTHERMODYNAMICS
NCERT FINGERTIPS ENGLISH|Exercise ASSERTION & REASON|15 VideosTHERMODYNAMICS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosTHE S-BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-THERMODYNAMICS-HIGHER ORDER THINKING SKILLS
- A ideal gas does work on its surroundings when it expands by 2.5 L aga...
Text Solution
|
- A given mass of gas expands from state A to state B by three paths 1, ...
Text Solution
|
- For an ideal gas, consider only P-V work in going from an initial stat...
Text Solution
|
- H(2) gas is mixed with air at 25^(@)C under a pressure of 1 atmosphere...
Text Solution
|
- Bond dissociation enthalpies of H(2(g)) and N(2(g)) are "426.0 kJ mol...
Text Solution
|
- Consider the following two reactions : (i) "Propene "+H(2) rarr "Pro...
Text Solution
|
- A gaseous system is initially characterised by 500 mL volume and 1 atm...
Text Solution
|
- What will be DeltaG for the reaction at 25^(@)C when partial pressures...
Text Solution
|