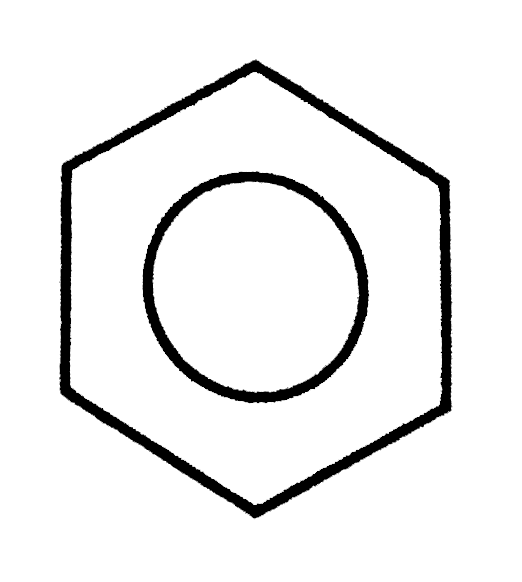
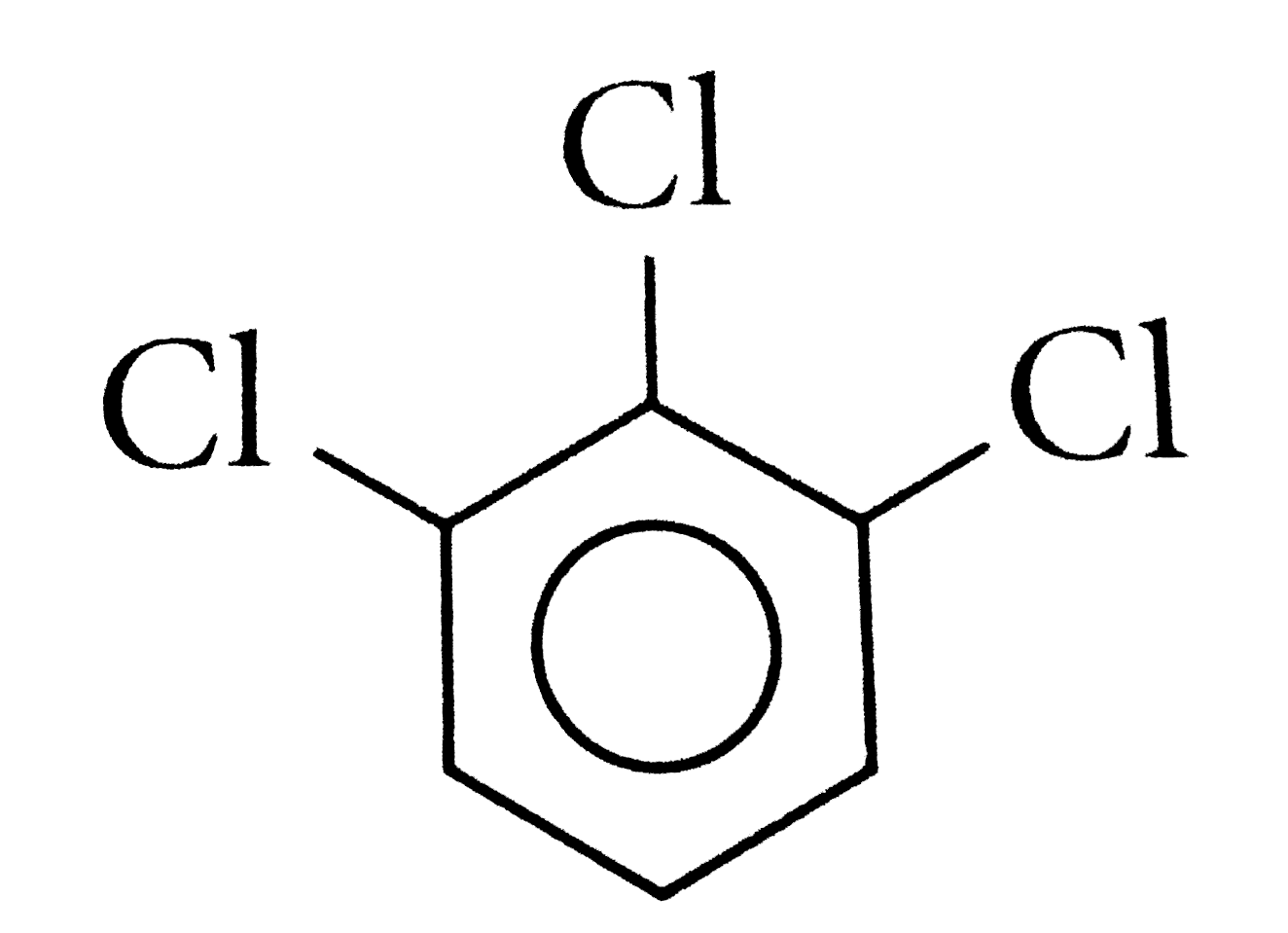
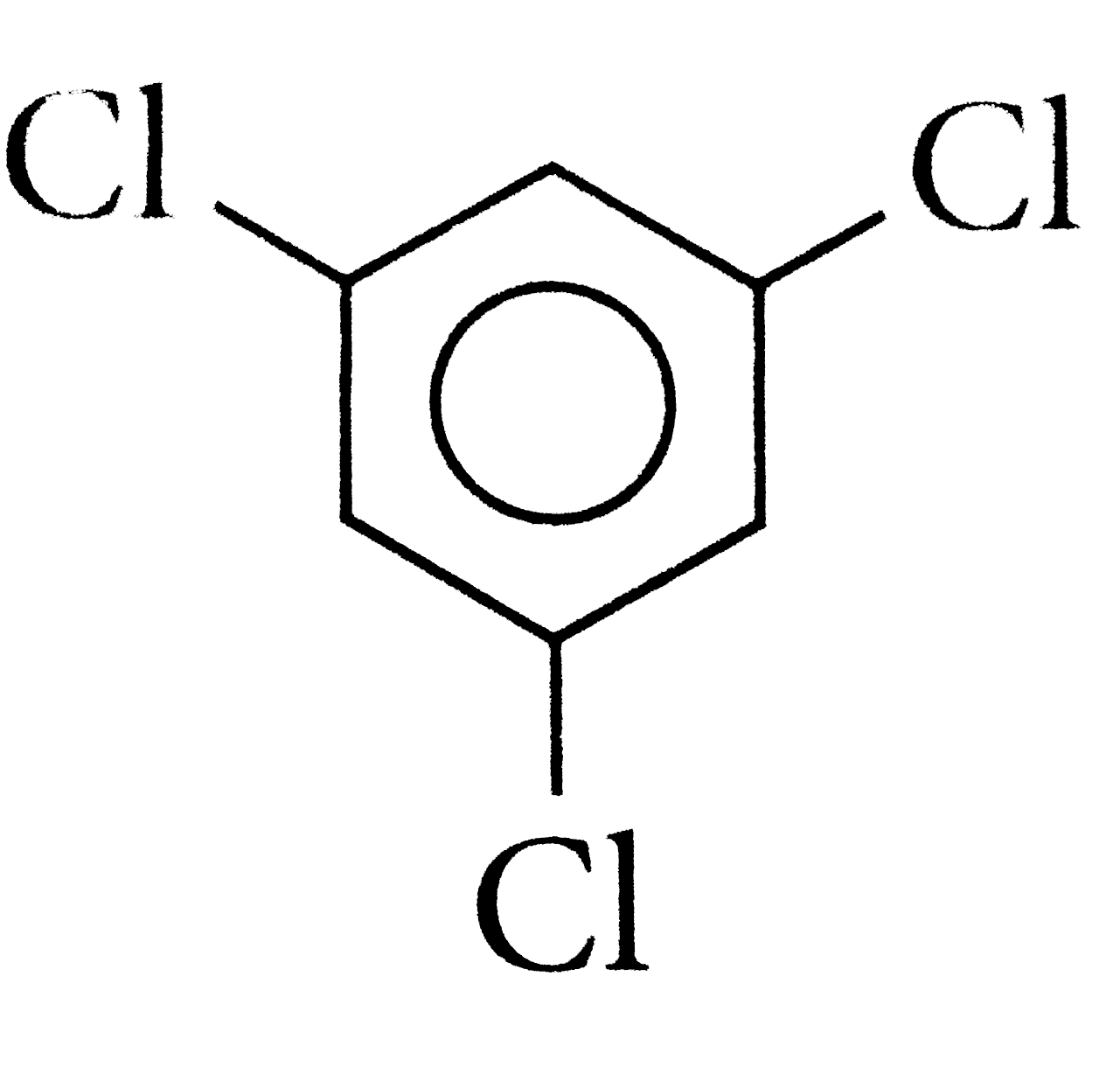
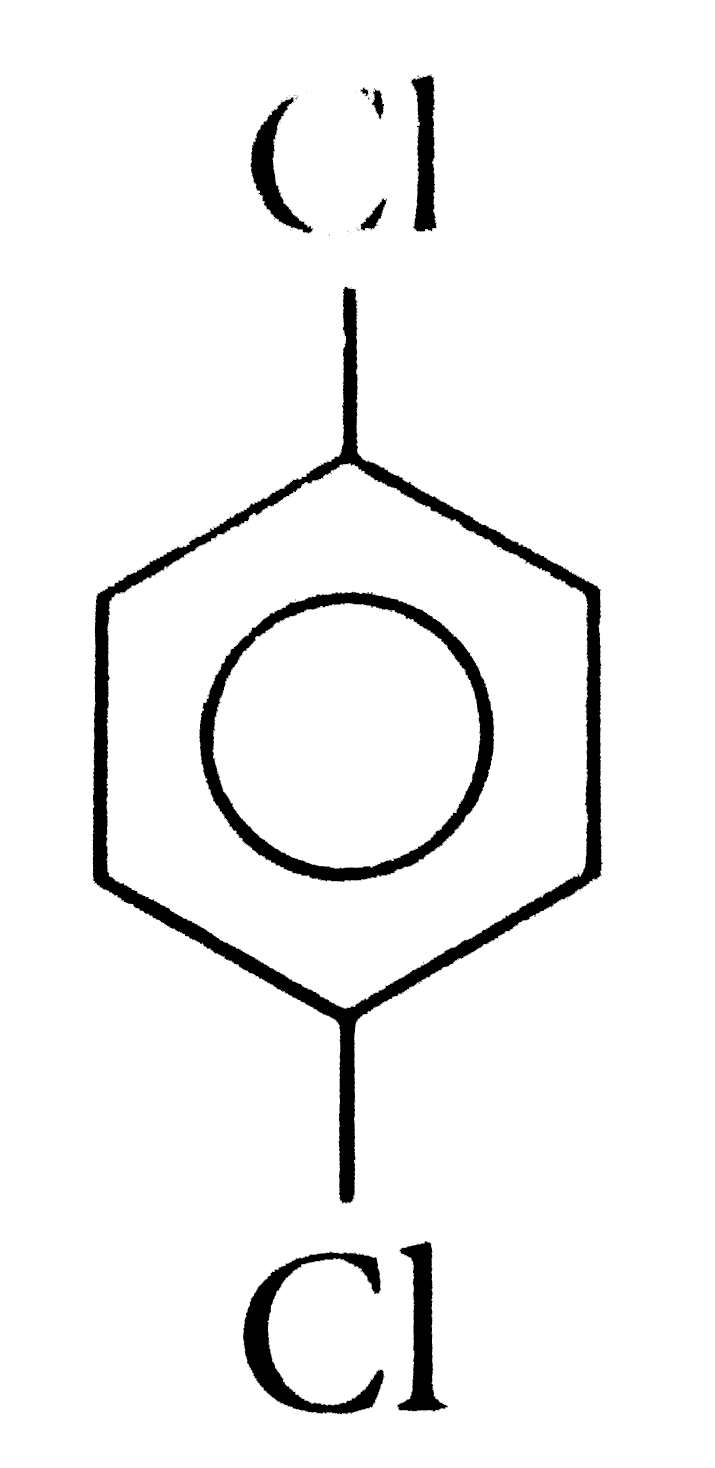
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-PRACTICE PAPER 3-Practice Paper 3
- Select correct statement for BrF(5).
Text Solution
|
- Consider a P(y) orbital of an atom and identify correct statement
Text Solution
|
- Which of the following will have maximum dipole moment?
Text Solution
|
- Which of the following is not the consequence of H-bonding?
Text Solution
|
- The two equilibrium AB hArr A^(+) + B^(-) and AB+B^(-)hArrAB(2)^(-) ar...
Text Solution
|
- Consider the following equilibrium in a closed container, N(2)O(4(g)...
Text Solution
|
- The degree of dissociation alpha of the reaction" N(2)O(4(g))hArr 2N...
Text Solution
|
- (I) H(2)O(2)+O(3) to H(2)O+2O(2) (II) H(2)O(2)+Ag(2)O to 2Ag+H(2)O+O...
Text Solution
|
- Which set of quantum numbers is possible for the last electron of Mg^(...
Text Solution
|
- Which of the following reactions is said to be entropy driven?
Text Solution
|
- If 10^(21) molecules are removed from 200 mg of CO(2), the number of m...
Text Solution
|
- The ions O^(-2),F^(-),Mg^(2+) and Al^(3+) are isoelectronic. Their ion...
Text Solution
|
- The pH of 0.004 M hydrazine solution is 9.7. its ionisation constant (...
Text Solution
|
- The vapoour density of a mixture containing NO(2) and N(2)O(4) is 38.3...
Text Solution
|
- An alkane C(7)H(16) is produced by the reaction of lithium di(3-pentyl...
Text Solution
|
- The enthalpy of neutralisation of NH(4)OH and CH(3)COOH is -10.5 kcal ...
Text Solution
|
- When LiNO(3) is heated, it gives oxide, Li(2)O whereas other alkali m...
Text Solution
|
- Which one of the following statements is not true?
Text Solution
|
- The solubility product of MgF(2) is 7.4xx10^(-11). Calculate the solub...
Text Solution
|
- The aqueous solution of potash alum [K(2)SO(4)*Al(2)(SO(4))(3)*24H(2)O...
Text Solution
|