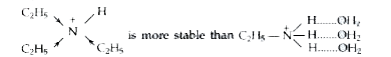(i) In `C_(6)H_(5)NH_(2)`, the lone pair of electrons on the N-atom is delocalised over the benzene ring. In contrast, N in `C_(6)H_(5)CH_(2)NH_(2)` is not directly linked to the benzene ring and hence its lone pair is not delocalised over the benzene ring. In other words, the lone pair of electrons on the N-atom in `C_(6)H_(5)CH_(2)NH_(2)` is more easily available than that on the N-atom in `C_(6)H_(5)NH_(2)`. Thus, `C_(6)H_(5)CH_(2)NH_(2)` is more basic than `C_(6)H_(6)NH_(2)`. Due to +I-effect of `C_(2)H_(5)` group, `C_(2)H_(5)NH_(5)` is more basic than `C_(6)H_(5)CH_(2)NH_(2)`. However, both of them are stronger bases than `NH_(3)`. Further due to +I- effect of the two `C_(2)H_(5)` groups in `(C_(2)H_(5))_(2)NH` as compared to one in `C_(2)H_(5)NH_(2), (C_(2)H_(5))_(2)NH` is a stronger base than `C_(2)H_(5)NH_(2)`. Thus, the basic strength increases in the order :
`" "C_(6)H_(5)NH_(2) lt NH_(3) lt C_(6)H_(5)CH_(2)NH_(2) lt C_(2)H_(5)NH_(2) lt (C_(2)H_(5))_(2)NH`
(ii) In `C_(2)H_(5)NH_(2), (C_(2)H_(5)NH" and "(C_(2)H_(5))_(3)N`, the +I-effect of the `C_(2)H_(5)` group/s increases the electron density on the N-atom. However, in `C_(6)H_(5)NH_(2)`, the electron density on the N-atom is smaller due to delocalisation of the lone pair of electrons over the benzene ring. Therefore, all the three ethylamines are more basic than `C_(6)H_(5)NH_(2)`.
In aqueous solution the relative basic strength of `C_(2)H_(5)NH_(2), (C_(2)H_(5))_(2)NH" and "(C_(2)H_(5))_(3)` depends upon the stabilisation of their cations (formed as a result of accepting a proton from water) by a number of factors such as H-bonding, steric hindrance of the alkyl groups and +I-effect of the alkyl groups. All these factors are favourable for `2^(@)` amines, therefore, `(C_(2)H_(5))_(2)NH` is a stronger base than `C_(2)H_(5)NH_(2)" and "(C_(2)H_(5))_(3)N`. Since `C_(2)H_(5)` group is bigger, it exerts some steric hindrance to H-bonding. Therefore, stabilisaton of the cation derived from `(C_(2)H_(5))_(3)N` due to +I-effect is greater than the stablisation of the acid derived from `C_(2)H_(5)NH_(2)` by H-bonding, i.e.,
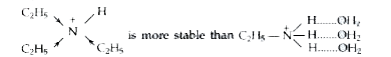
Therefore, `(C_(2)H_(5))_(3)N` is more basic than `C_(2)H_(5)NH_(2)`. Thus, the basic strength of the four amines increases in the order :
`" "C_(6)H_(5)NH_(2) lt C_(2)H_(5)NH_(2) lt (C_(2)H_(5))_(3)N lt (C_92)H_(5))_(2)NH`.
(iii) Due to greater availability of electrons, `C_(6)H_(5)CH_(2)NH_(2)` is more basic than `C_(6)H_(5)NH_(2)`. Due to +I-effect of the `CH_(3)` groups, the electrons density on the N-atom in `CH_(3)NH_(2), (CH_(3))_(2)NH" and "(CH_(3))_(3)N` increases. However, in `C_(6)H_(5)NH_(2)" and "C_(6)H_(5)CH_(2)NH_(2)` electron density on the N-atom is smaller due to electron-withdrawing resonance effect of the `C_(6)H_(5)` group in `C_(6)H_(5)NH_(2)` and due to electron-withdrawing inductive effect (i.e., -I-effect) of the `C_(6)H_(5)` group in `C_(6)H_(5)CH_(2)NH_(2)`. Therefore, all the three methylamines are more basic than `C_(6)H_(5)NH_(2)` and `C_(6)H_(5)CH_(2)NH_(2)`.
Basic strengths of `CH_(3)NH_(2), (CH_(3))_(2)NH" and "(CH_(3))_(3)N` depends upon the stabilisation of their cations (formed as a result of accepting a proton from `H_(2)O`) by a number of factors such as H-bonding, steric hindrance of the alkyl groups and +I-effect of the alkyl groups. All these factors are favourable for `2^(@)` amines, therefore, `(CH_(3))_(2)NH` is a stronger base than `CH_(3)NH_(2)" and "(CH_(3))_(3)N`. Stabilisation of the cation derived from `CH_(3)NH_(2)` due to H-bonding is greater because of one `CH_(3)` group than that of the conjugate acid derived from `(CH_(3))_(3)N` due to the presence of three `CH_(3)` groups. Thus,

Therefore, `CH_(3)NH_(2)` is a stronger base than `(CH_(3))_(3)N`. The overall basic strength of the five amines is in the order :
`" "C_(6)H_(5)NH_(2) lt C_(6)H_(5)CH_(2)NH_(2) lt (CH_(3))_(3)N lt CH_(3)NH_(2) lt (CH_(3))_(2)NH`