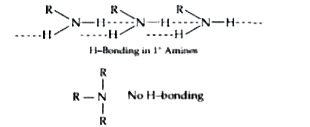
Text Solution
Verified by Experts
Topper's Solved these Questions
AMINES
U-LIKE SERIES|Exercise CASE BASED/SOURCE-BASED INTEGRATED QUESTIONS|14 VideosAMINES
U-LIKE SERIES|Exercise MULTIPLE CHOICE QUESTIONS|20 VideosAMINES
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST|6 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST (SECTION B)|1 VideosBIOMOLECULES
U-LIKE SERIES|Exercise LONG ANSWER QUESTIONS - I|22 Videos
Similar Questions
Explore conceptually related problems
U-LIKE SERIES-AMINES-NCERT TEXTBOOK EXERCISES
- Accomplish the following conversions : Aniline to benzyl alcohol.
Text Solution
|
- Give the structures of A, B and C in the following reactions : CH(3)...
Text Solution
|
- Give the structures of A, B and C in the following reactions : C(6)H...
Text Solution
|
- Give the structures of A, B and C in the following reactions : CH(3)...
Text Solution
|
- Give the structures of A, B and C in the following reactions : C(6)H...
Text Solution
|
- Give the structures of A, B and C in the following reactions : CH(3)...
Text Solution
|
- Give the structures of A, B and C in the following reactions : C(6)H...
Text Solution
|
- An aromatic compound 'A' on treatment with aqueous ammonia and heating...
Text Solution
|
- Complete the following reactions : C(6)H(5)NH(2)+CHCl(3)+alc. KOH ra...
Text Solution
|
- Complete the following reactions : C(6)H(5)N(2)Cl+H(3)PO(2)+H(2)O ra...
Text Solution
|
- Complete the following reactions : C(6)H(5)NH(2)+H(2)SO(4) (conc.) r...
Text Solution
|
- Complete the following reactions : C(6)H(5)N(2)Cl+C(2)H(5)OH rarr
Text Solution
|
- Complete the following reactions : C(6)H(5)NH(2)+Br(2) (aq) rarr
Text Solution
|
- Complete the following reactions : C(6)H(5)NH(2)+(CH(3)CO)(2)O rarr
Text Solution
|
- Complete the following reactions : C(6)H(5)N(2)Cl underset((ii)NaNO(...
Text Solution
|
- Why cannot aromatic primary amines be prepared by Gabriel phthalimide ...
Text Solution
|
- Write the reactions of (i) aromatic and (ii) aliphatic primary amines ...
Text Solution
|
- Give plausible explanation for each of the following : Why ae amines...
Text Solution
|
- Give plausible explanation for each of the following : Why do primar...
Text Solution
|
- Give plausible explanation for each of the following : Why are aliph...
Text Solution
|