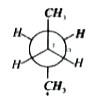
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ISOMERISM
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE-II (MORE THAN ONE CORRECT TYPE QUESTIONS))|3 VideosISOMERISM
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE-II (LINKED COMPREHENSION TYPE QUESTIONS))|3 VideosISOMERISM
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE-I (INTEGER TYPE QUESTIONS))|3 VideosIONIC EQUILIBRIUM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL -II PRACTICE SHEET (ADVANCED) (Integer Type Questions))|8 VideosPERIODIC CLASSIFICATION
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (RECENT AIPMT/NEET QUESTIONS)|14 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ISOMERISM -LECTURE SHEET (EXERCISE-II (STRAIGHT OBJECTIVE TYPE QUESTIONS))
- In the given conformation C(2) is rotated about C(2)-C(3) bond by 120^...
Text Solution
|
- The number of conformational isomers possible of n-butane about C(2)-C...
Text Solution
|
- Which of the following is correct regarding stability of conformations...
Text Solution
|
- Which of the following is the most stable conformer of 1, 2, 4 -trimet...
Text Solution
|
- Choose the stable form of the compound given below in chair conformati...
Text Solution
|
- Translating this eclipsed Newman projection formula of 2, 3 dibromobut...
Text Solution
|
- Geometrical isomerism is possible about which of the following multipl...
Text Solution
|
- Which of the following possess highest melting point
Text Solution
|
- Number of possible geometrical isomers for 2, 4 - hexadjenie is
Text Solution
|
- Which of the following statements is right regarding geometrical isome...
Text Solution
|
- The IUPAC name of the compound given is
Text Solution
|
- Which of the following order of stability is correct among these two i...
Text Solution
|
- Which of the following does not show geometrical isomerism?
Text Solution
|
- The name of the molecule shown is
Text Solution
|
- Identify the pair of isomers which are geometrical isomers of each.
Text Solution
|
- Which of the following cycloalkanes will show cis-trans isomerisin?
Text Solution
|
- Which of the following compounds is Z-isomer
Text Solution
|
- This compound is named in IUPAC as
Text Solution
|
- CH(2)=CH-CH=CH-CH=CH-CH(3) How many geometrical isomers of this compou...
Text Solution
|
- How many geometrical isomers of this compound are possible?
Text Solution
|