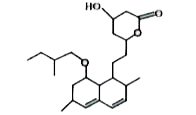
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ISOMERISM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE ( PRACTICE SHEET (ADVANCED) MORE THAN ONE CORRECT ANSWER TYPE QUESTIONS)|14 VideosISOMERISM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE ( PRACTICE SHEET (ADVANCED) LINKED COMPREHENSION TYPE QUESTIONS)|8 VideosISOMERISM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL-II LECTURE SHEET (ADVANCED) MATRIX MATCHING TYPE QUESTIONS)|3 VideosIONIC EQUILIBRIUM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL -II PRACTICE SHEET (ADVANCED) (Integer Type Questions))|8 VideosPERIODIC CLASSIFICATION
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (RECENT AIPMT/NEET QUESTIONS)|14 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ISOMERISM -ADDITIONAL PRACTICE EXERCISE (LEVEL-II LECTURE SHEET (ADVANCED) INTEGER TYPE QUESTIONS)
- How many sterogenic centers are there in Lovastain (Mevacor(R): a chol...
Text Solution
|
- Identify how many chiral atoms are present in the following well known...
Text Solution
|
- How many steriogenic centre is present for the given structure
Text Solution
|
- Butaclamol is potent antipsychotic that has been used clinically in th...
Text Solution
|
- No. of possible isomeric dioic acids for the molecular formulae C(4)H(...
Text Solution
|
- No. of compounds having net dipole moment are
Text Solution
|
- Find the number of enantiomeric pairs that can be produced during the ...
Text Solution
|
- How many structural cyclic isomers of C(5)H(10) are possible?
Text Solution
|
- The minimum number of carbon atoms to be present in a carboxylic acid ...
Text Solution
|
- Find the possible number of stereoisomers formed in the following reac...
Text Solution
|
- How many spatial orientations are possible in the following compound ?
Text Solution
|