A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DC PANDEY ENGLISH-CURRENT ELECTRICITY-All Questions
- Spheres P and Q are uniformally constructed from the same material whi...
Text Solution
|
- A container X has volume double that of container Y and both are conne...
Text Solution
|
- A givne mass of a gas expands from a state A to the state B by three p...
Text Solution
|
- The figure shows the graph of logarithmic reading of pressure and volu...
Text Solution
|
- An ideal monoatomic gas is carried around the cycle ABCDA as shown in ...
Text Solution
|
- In a thermodynamic process, pressure of a fixed mass of gas is changed...
Text Solution
|
- The relation between U, p and V for an ideal gas in an adiabatic proce...
Text Solution
|
- A thermal insulated vessel contains some water at 0^(@)C. The vessel i...
Text Solution
|
- The temperature drop through each layer of a two layer furnace wall is...
Text Solution
|
- Three different arrangemnets of matrials 1 and 2,3 to from a wall Thre...
Text Solution
|
- On a pT diagram, a cyclic process is performed as shown. Where is the ...
Text Solution
|
- A monoatomic ideal is used as the working substance for the carnot cyc...
Text Solution
|
- Figure illustrates a cycle conducted with n moles of an ideal gas. In ...
Text Solution
|
- One mole of a diatomic gas undergoes a process P = P(0)//[1 + (V//V(0)...
Text Solution
|
- 3 moles of an ideal mono atomic gas performs a cycle as shown in fig. ...
Text Solution
|
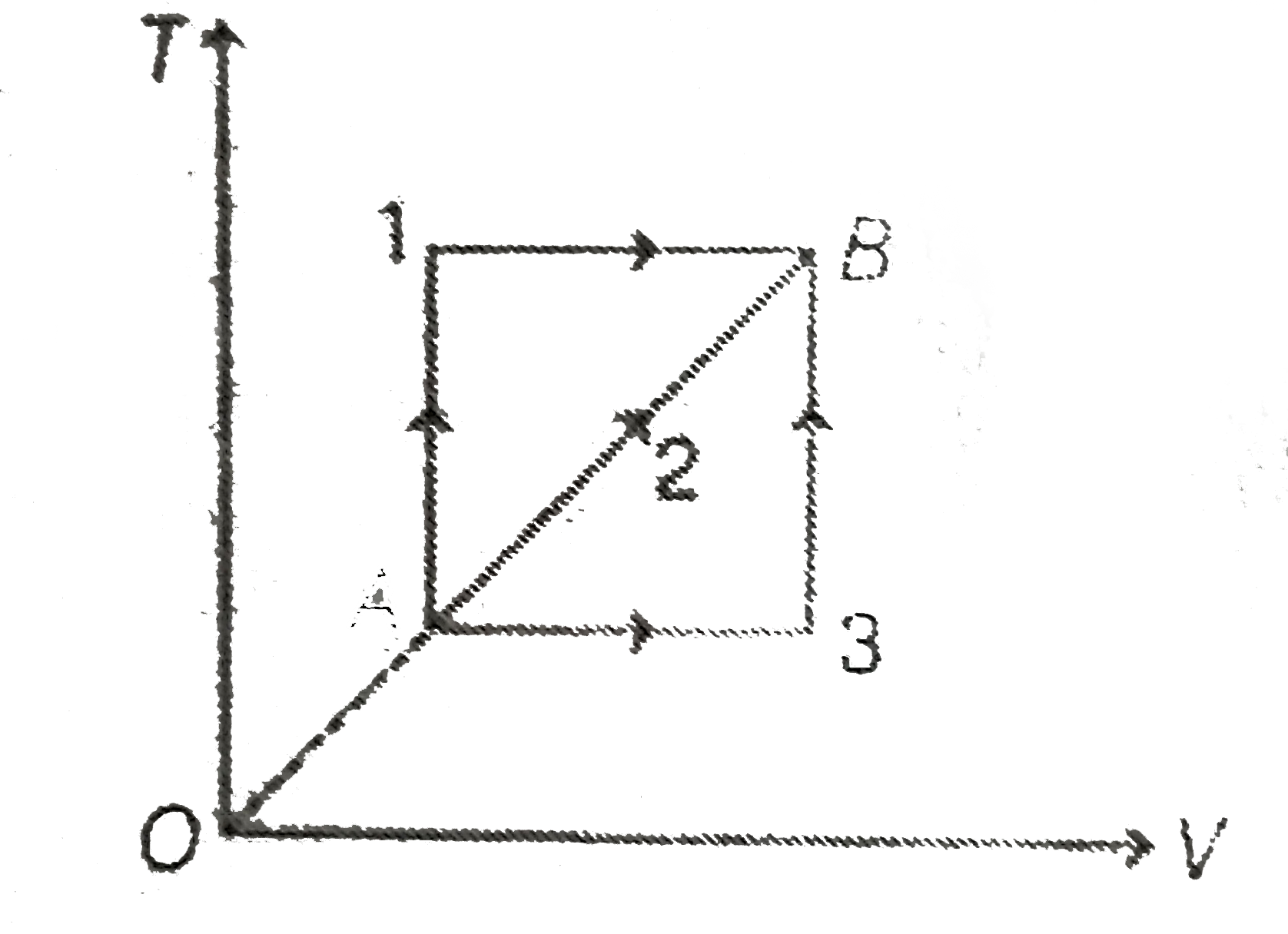
- Given T-p curve for three processes. Work done in process 1, 2 and 3 (...
Text Solution
|
- Figure shows two flasks connected to each other. The volume of the fla...
Text Solution
|
- A cylinder piston of mass M sides smoothlly inside a long cylinder clo...
Text Solution
|
- A 1-L flask contains some mercury. It is found that at different tempe...
Text Solution
|
- A point source of heat of power P is placed at the centre of a spheric...
Text Solution
|