Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK ELEMENTS
ICSE|Exercise EXERCISE (PART-II)(DESCRIPTIVE QUESTIONS)(SHORT ANSWER QUESTIONS)|67 VideosP-BLOCK ELEMENTS
ICSE|Exercise EXERCISE (PART-II)(DESCRIPTIVE QUESTIONS)(LONG ANSWER QUESTIONS)|65 VideosP-BLOCK ELEMENTS
ICSE|Exercise EXERCISE (PART-I)(MATCH THE FOLLOWING)|1 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
ICSE|Exercise ISC EXAMINATION QUESTIONS ( PART-II DESCRIPTIVE QUESTIONS)|33 VideosPOLYMERS
ICSE|Exercise ISC EXAMINATION QUESTIONS |10 Videos
Similar Questions
Explore conceptually related problems
ICSE-P-BLOCK ELEMENTS-EXERCISE (PART-II)(DESCRIPTIVE QUESTIONS)(VERY SHORT ANSWER QUESTIONS)
- What is tailing of mercury ?
Text Solution
|
- What is ozonolysis ?
Text Solution
|
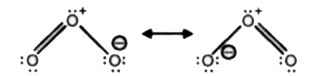
- Why is O-O bond length in ozone (128 pm) more than in O2 (122 pm)?
Text Solution
|
- Why ozone is used in improving the atmosphere of crowded places such a...
Text Solution
|
- Write balanced chemical equations for the preparation of SO2 from a s...
Text Solution
|
- Why quicklime is not used for drying SO2?
Text Solution
|
- What is produced when SO2 is dissolved in water?
Text Solution
|
- Name one substance which can be used as a catalyst in the formation of...
Text Solution
|
- Write one chemical equation to show that SO2 acts as a reducing agent...
Text Solution
|
- What happens when SO2 reacts with Cl2 : (i) in presence of sunlight....
Text Solution
|
- SO2 acts as a bleaching agent. How?
Text Solution
|
- Dry SO(2) does not bleach dry flowers because
Text Solution
|
- Why does SO2 exhibit bleaching action only in presence of water?
Text Solution
|
- What happens when H2S reacts with SO2 ?
Text Solution
|
- What happens when SO2 is passed through caustic soda solution ?
Text Solution
|
- What is the bond angle in SO2 ?
Text Solution
|
- State the name of anhydride of H2SO4.
Text Solution
|
- Why sulfuric acid is described as king of chemicals ?
Text Solution
|
- Why sulfuric acid is also known as oil of vitriol?
Text Solution
|
- Why the process of manufacture of H2SO4 is named contact process ?
Text Solution
|