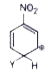
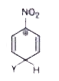
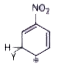
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
ICSE|Exercise TRUE OR FALSE TYPE QUESTIONS|50 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
ICSE|Exercise FILL IN THE BLANK TYPE QUESTIONS |46 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
ICSE|Exercise ESSAY ( LONG ANSWER ) TYPE QUESTIONS|42 VideosHYDROGEN
ICSE|Exercise NCERT TEXT-BOOK EXERCISE (With Hints and Solutions)|53 VideosREDOX REACTIONS (OXIDATION AND REDUCTION)
ICSE|Exercise NCERT TEXT-BOOK. EXERCISES ((With Hints and Solutions)|69 Videos
Similar Questions
Explore conceptually related problems
ICSE-ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES -OBJECTIVE ( MULTIPLE CHOICE ) TYPE QUESTIONS
- Which of the following has the highest nucleophilicity ? F^(-) OH^...
Text Solution
|
- The order of reactivities of the following alkyl halides for an S(N^2)...
Text Solution
|
- Following reaction, (CH3)3 CBr +H2O to (CH3)3 COH +HBr is an ...
Text Solution
|
- Due to the presence of an unpaired electron, free radicals are
Text Solution
|
- The organic chloro compound, which shows complete stereochemical inve...
Text Solution
|
- Which one is a nucleophilic substitution reaction among the following ...
Text Solution
|
- The order of stability of the following carbocations CH2 = underset...
Text Solution
|
- The correct statement regarding electrophile is
Text Solution
|
- The most suitable method of separation of 1:1 mixture of ortho and par...
Text Solution
|
- Identify A and predict the type of reaction
Text Solution
|
- The IUPAC name of the compound
Text Solution
|
- Which of the following is correct with respect to -|-effect of the sub...
Text Solution
|
- Which of the following carbocations is expected to be most stable?
Text Solution
|
- Which of the following compounds will be suitable for Kjeldahl's metho...
Text Solution
|
- The number of sigma (sigma ) and pi (pi) bonds in pent-2-en-4-yne is :
Text Solution
|
- The IUPAC name for the following compound is :
Text Solution
|
- Increasing order of reactivity of the following compounds for S(N^1) s...
Text Solution
|
- Which of the following is potential energy diagram for S(N)1 reaction?
Text Solution
|
- The correct IUPAC name of the following compound is :
Text Solution
|
- The IUPAC name of the following compound is: H(3)C-overset(CH(3))ove...
Text Solution
|