Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-THERMODYNAMICS-QUESTION BANK
- Two litres of an ideal gas at a pressure of 10 atm expands isothermall...
Text Solution
|
- Two litres of an ideal gas at a pressure of 10 atm expands isothermall...
Text Solution
|
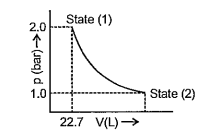
- 1.0 mole of a monoatomic ideal gas is expanded from state (1) to state...
Text Solution
|
- One mole of an ideal gas at STP is heated to 27^@C and compressed to 1...
Text Solution
|
- Calculate heat of the following reaction at constant pressure, F2O(g) ...
Text Solution
|
- If water vapour is assumed to be a perfect gas and molar enthalpy chan...
Text Solution
|
- If water vapour is assumed to be a perfect gas and molar enthalpy chan...
Text Solution
|
- The enthalpy of combustion of methane, graphite & dihydrogen at 298 K ...
Text Solution
|
- Heat of reaction for, C6H(12)(s) + 6O2(g) to 6 CO2(g)+6H2O(l) at co...
Text Solution
|
- Calculate the number of kJ of heat necessary to raise the temperature ...
Text Solution
|
- Enthalpies of formation of CO(g),CO2(g),N2O(g) and N2O4(g) are -110,-3...
Text Solution
|
- Calculate the enthalpy change on freezing of 1.0 mole of water at 10^@...
Text Solution
|
- Calculate the enthaply change for the process : C Cl4(g) to C(g)+4Cl...
Text Solution
|
- For an isolated system, triangleU = 0, what will be triangleS ?
Text Solution
|
- Calculate the entropy change in surroundings when 1.00 mole of H2O(l) ...
Text Solution
|
- For the reaction 2A(g) + B(g) to 2D(g), triangleU^@ = -10.5kJ and tr...
Text Solution
|
- Predict in the given question, entropy increases/ decreases A liquid...
Text Solution
|
- Predict in the given question, entropy increases/ decreases 2NaHCO3(...
Text Solution
|
- Predict in the given question, entropy increases/ decreases H2(g) to...
Text Solution
|
- For oxidation of Fe 4Fe(s) + 3O2(g) to 2Fe2O3(s) entropy change is -5...
Text Solution
|