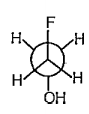
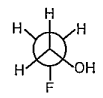
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-GOC-II & III-QUESTION BANK
- In crystal arrangement of NaCl, the arrangement of Cl− ion is
Text Solution
|
- Among above structure find out enantiomeric sturctures :
Text Solution
|
- Conformations are different arrangements of atoms that are interconver...
Text Solution
|
- Conformations are different arrangements of atoms that are interconver...
Text Solution
|
- Which one has the highest reactivity towards nucleophilic addition : ...
Text Solution
|
- Write the increasing order of reactivity towards the nucleophilic addi...
Text Solution
|
- Arrange the following carbonyl compounds in decreasing order of reacti...
Text Solution
|
- A solution prepared by mixing 10 mL of a 0.10 M solution of the R enan...
Text Solution
|
- Which of the following compounds will exhibit geometrical isomerism?
Text Solution
|
- In a mixture, two enantiomers are found to be present in 85% and 15% r...
Text Solution
|
- Two possible stereo-structures of CH3CHOHCOOH,which are optically acti...
Text Solution
|
- A solution of (-) 1-chloro-1-phenylethane in toluene racemises slowly ...
Text Solution
|
- Dissymmetric object is one which is
Text Solution
|
- The compound without a chiral carbon atom is
Text Solution
|
- In SN1 reaction, the racemization takes place. It is due to
Text Solution
|
- Which of the following pairs of compounds are enantiomers ?
Text Solution
|
- How many compounds are possible on monochlorination of butane ?
Text Solution
|
- Which of the following is a chiral molecule ?
Text Solution
|
- Ibuprofen contains
Text Solution
|
- The well known compounds, (+) -lactic acid and (-) -lactic acid, have ...
Text Solution
|