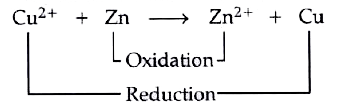Text Solution
Verified by Experts
Topper's Solved these Questions
STUDY OF THE FIRST ELEMENT - HYDROGEN
ICSE|Exercise TOPIC 2 Properties of Hydrogen, Oxidation nd Reduction (5 MARKS QUESTIONS)|29 VideosSTUDY OF THE FIRST ELEMENT - HYDROGEN
ICSE|Exercise TOPIC 2 Properties of Hydrogen, Oxidation nd Reduction (2 MARKS QUESTIONS)|14 VideosSTUDY OF GAS LAWS
ICSE|Exercise TOPIC 2 (5 Marks Questions ) |18 VideosTHE LANGUAGE OF CHEMISTRY
ICSE|Exercise EXCERCISE 1(C ) (Correct the following statement)|21 Videos
Similar Questions
Explore conceptually related problems
ICSE-STUDY OF THE FIRST ELEMENT - HYDROGEN-TOPIC 2 Properties of Hydrogen, Oxidation nd Reduction (3 MARKS QUESTIONS)
- Is it essential that oxidation and reduction must occur side by side i...
Text Solution
|
- Give reasons : Hydrogen is collected by the downward displacement of ...
Text Solution
|
- Give reasons : A candle brought near the mouth of a jar containing ...
Text Solution
|
- Give reasons : Apparatus for laboratory preparation of Hydrogen shoul...
Text Solution
|
- (a)State the position of Hydrogen in the periodic table. (b)Explain...
Text Solution
|
- Comment on the dual position of Hydrogen in the periodic table.
Text Solution
|
- Hydrogen may be prepared in the laboratory by the action of a metal wi...
Text Solution
|
- Give one example of Liquid oxidising agent
Text Solution
|
- Give one example of each A gaseous substance which acts as an oxidis...
Text Solution
|
- Give one example of Most preferred element/metal for preparing hydroge...
Text Solution
|
- What are oxidising agents ? Give two chemical tests for oxidising age...
Text Solution
|
- Hydrogen is obtained by displacement from dilute hydrochloric acid ...
Text Solution
|
- Hydrogen is obtained by displacement from dilute hydrochloric acid ...
Text Solution
|
- (i) Write the equation for the laboratory preparation of hydrogen. ...
Text Solution
|