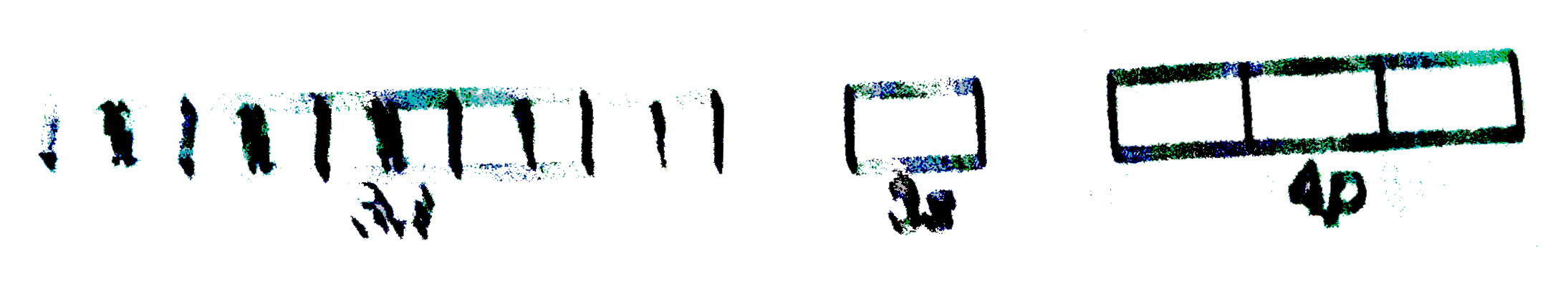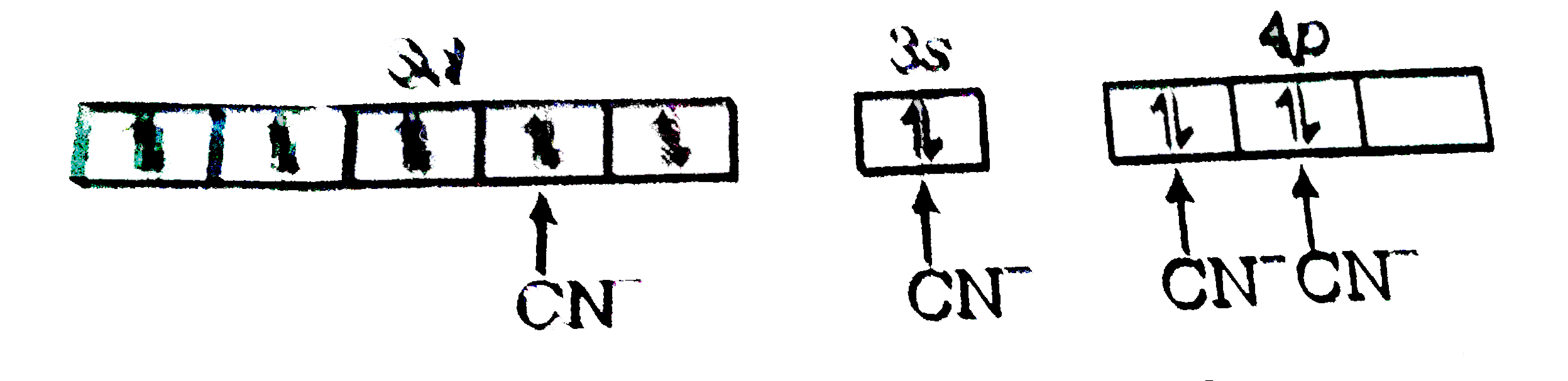A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The hybridization involved in complex [Ni(CN)(4)]^(2-) is (At. No . Ni...
Text Solution
|
- The hybridization involved in complex [Ni(CN)(4)]^(2-) is (At. No . Ni...
Text Solution
|
- Compare the following complexes with respect to structural shapes of u...
Text Solution
|
- [Ni(CN)(4)]^(2-) संकुल में संकरण है : [Ni का परमाणु क्रमांक = 28]
Text Solution
|
- The hybridization involved in the complex [Ni(CN)(4)]^(2-) is (At. No....
Text Solution
|
- Both [Ni(CO)(4)] and [Ni(CN)(4)]^(2-) are diamagnetic. The hybridizati...
Text Solution
|
- The hybridisation involved in complex [Ni(CN)(4)]^(2-) is ("At.no. of ...
Text Solution
|
- The hybridization involved in the complex [Ni(CN)(4)]^(2-) is…………
Text Solution
|
- The complex [Ni(CN)(4)]^(2-) is diamagnetic and it involves the follow...
Text Solution
|