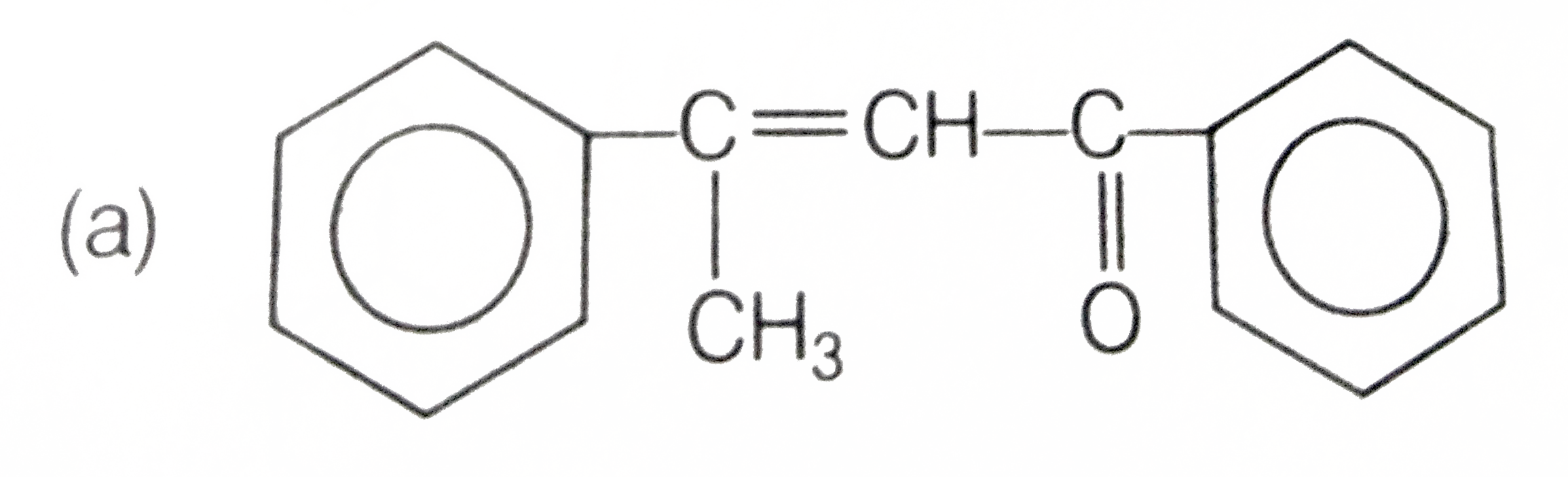
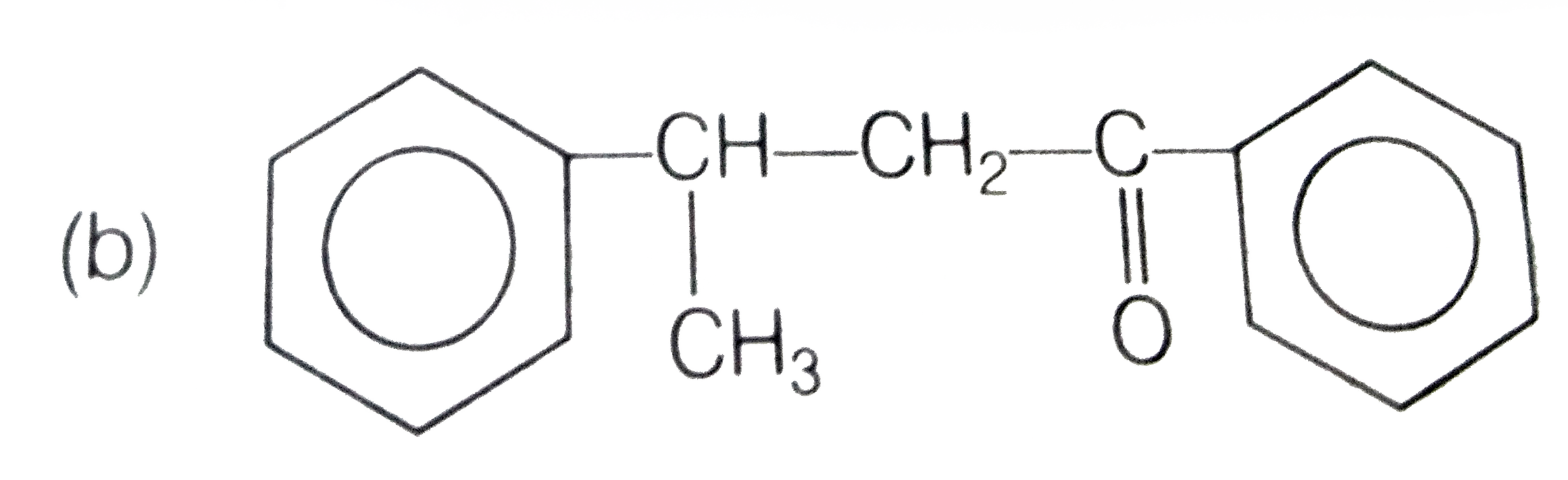
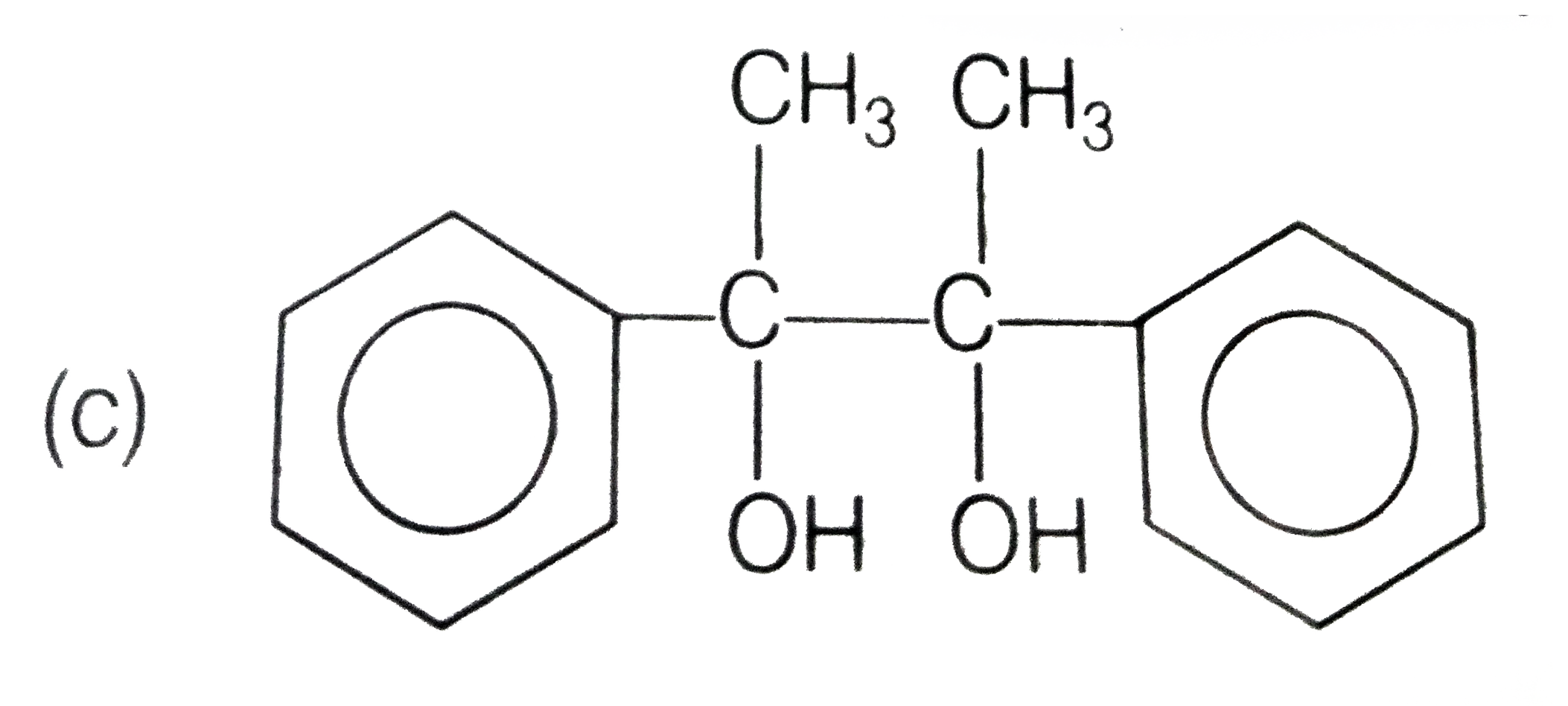
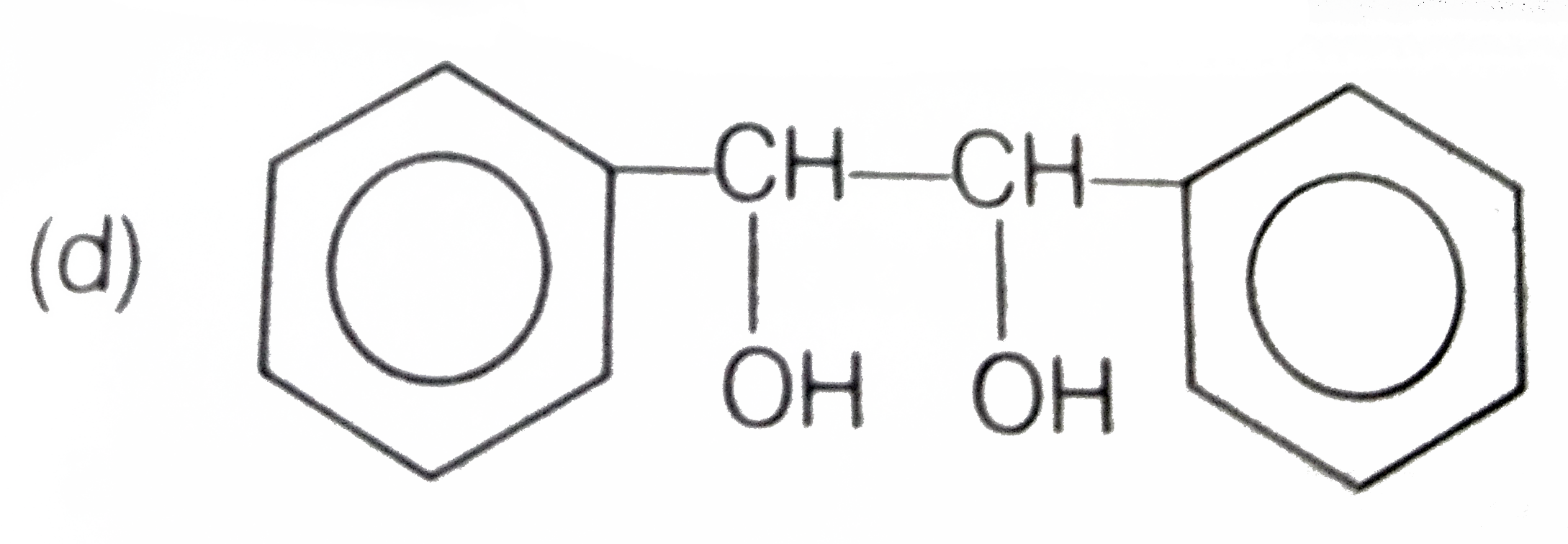
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Acetophenone when reacted with a base, C(2)H(5)ONa, yields a stable...
Text Solution
|
- Acetophenone when reacted with a base C(2)H(5)Ona yields a stable comp...
Text Solution
|
- 2 -Iodobutane on treatment with C(2)H(5)Ona//C(2)H(5)OH yields but - 2...
Text Solution
|
- Acetophenone when reacted with a base C(2)H(5)ONa yields a stable comp...
Text Solution
|
- In the given reaction maximum yield is obtained when (X) is: C(6)H(5)C...
Text Solution
|
- Acetophenone when reacted with a base, C(2)H(5)ONa, yields a stable co...
Text Solution
|
- Acetophenone when reacted with a base, C(2)H(5)ONa, yields a stable...
Text Solution
|
- Write the structure of the major organic product in each of the follow...
Text Solution
|
- ऐसिटोफीनोन को जब क्षार C(2)H(5)ONa के साथ अभिकृत कराया जाता है तो एक स...
Text Solution
|