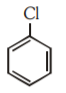
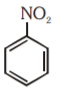
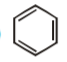
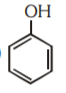
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following compounds is most reactive in electrophilic aro...
Text Solution
|
- Rank the following compounds in decreasing order of reactivity in elec...
Text Solution
|
- Arrange the following compound in reactivity towards EAS (Electrophili...
Text Solution
|
- The increasing order of reactivity of the following compounds towards ...
Text Solution
|
- The increasing order of reactivity of the following compounds towards ...
Text Solution
|
- Rank the following compounds in decreasing order of reactivity in elec...
Text Solution
|
- Which of the following is most reactive towards electrophillic aromat...
Text Solution
|
- Which of the following compounds is most reactive in electrophilic aro...
Text Solution
|
- The increasing order of the reactivity of the following compounds towa...
Text Solution
|