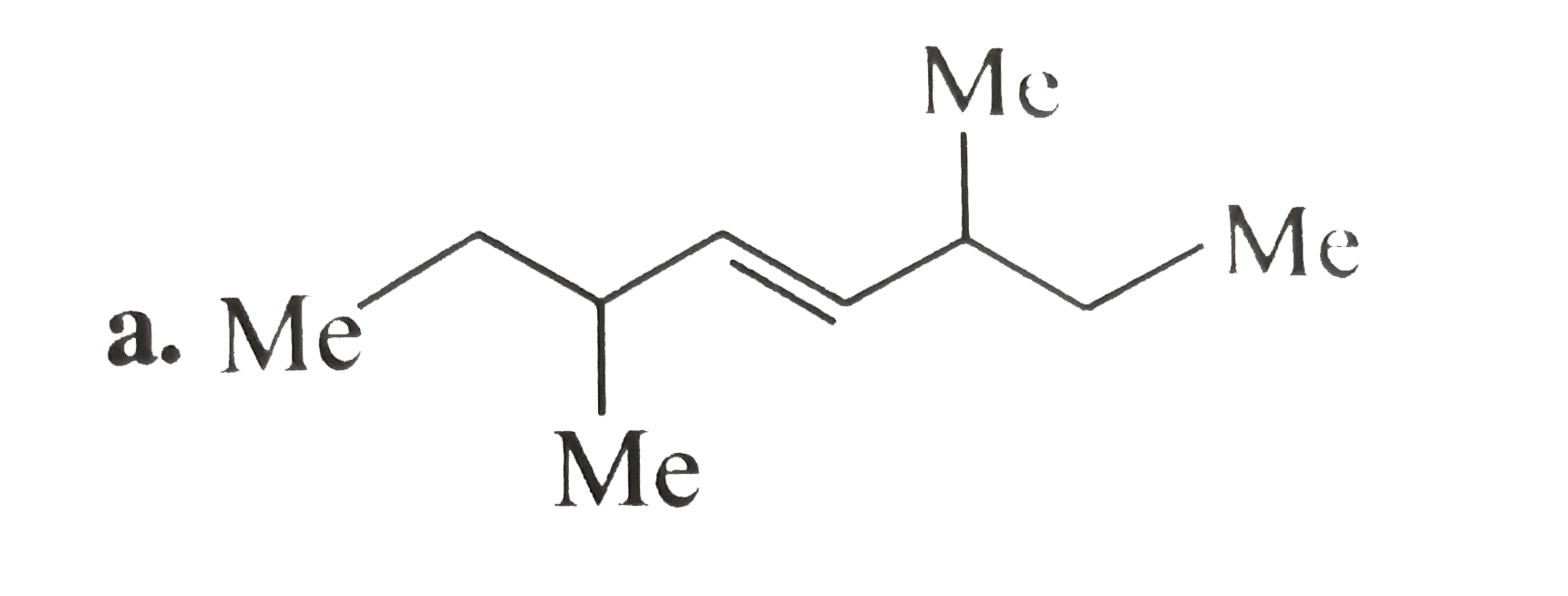
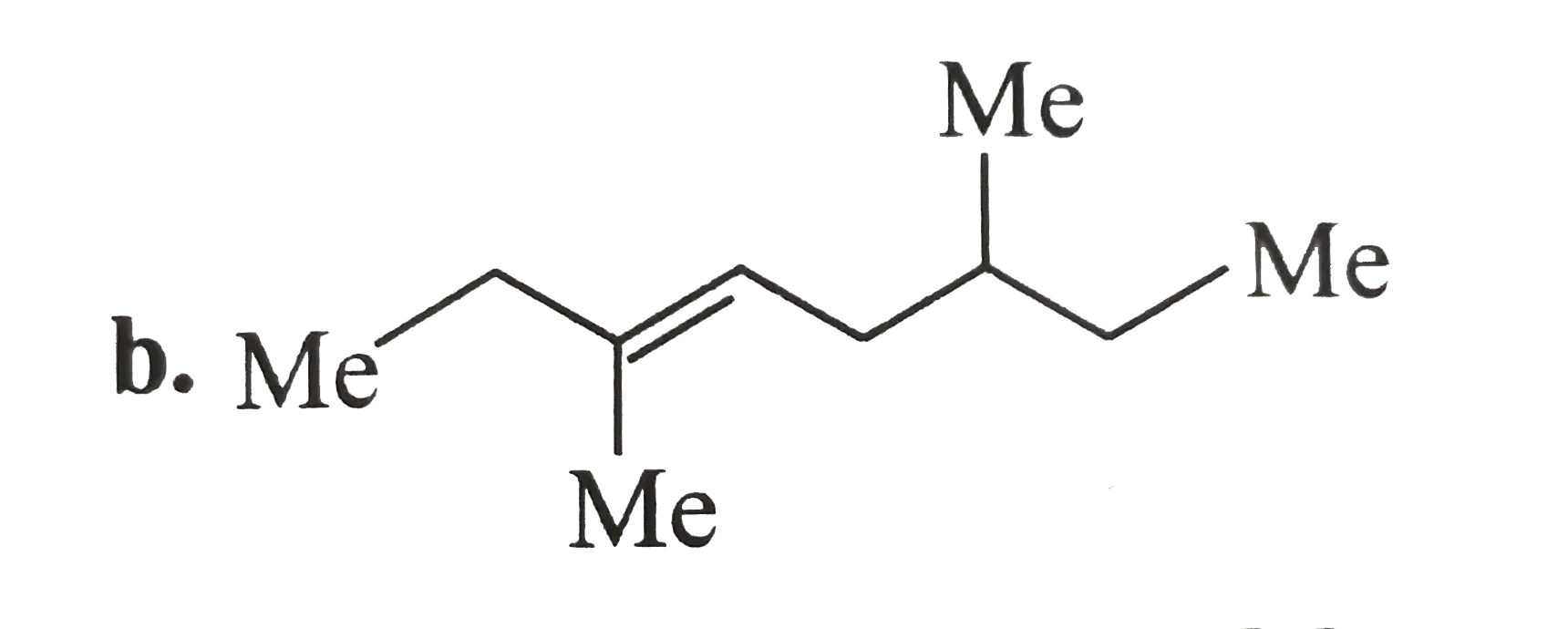
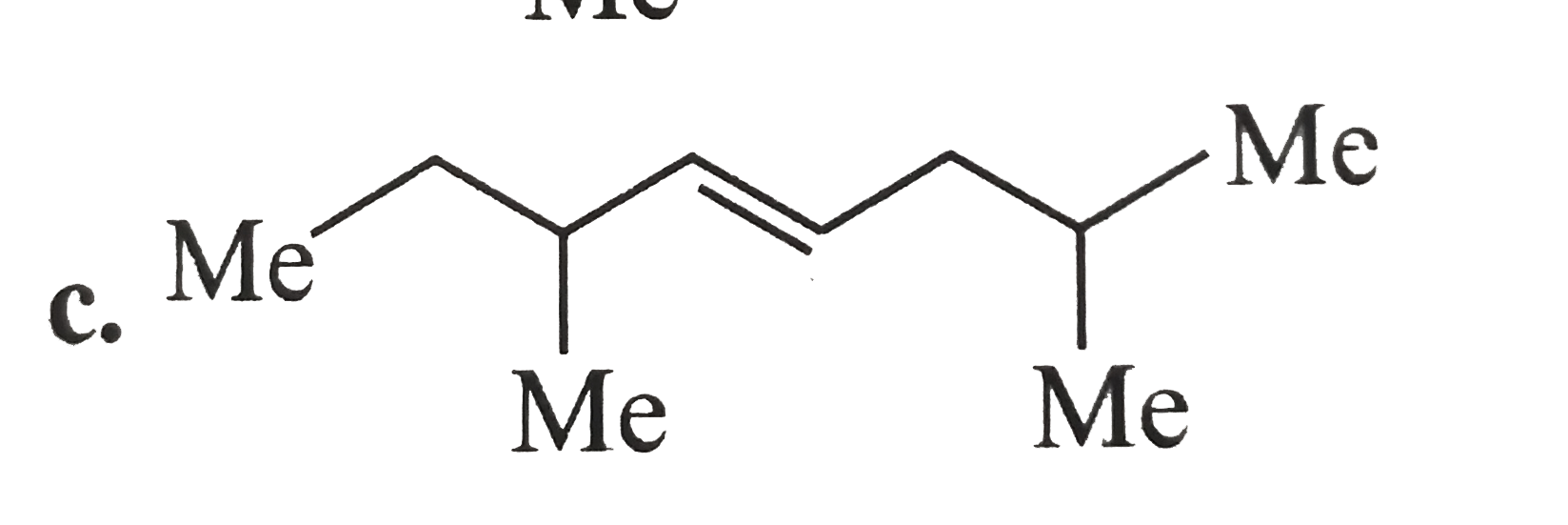
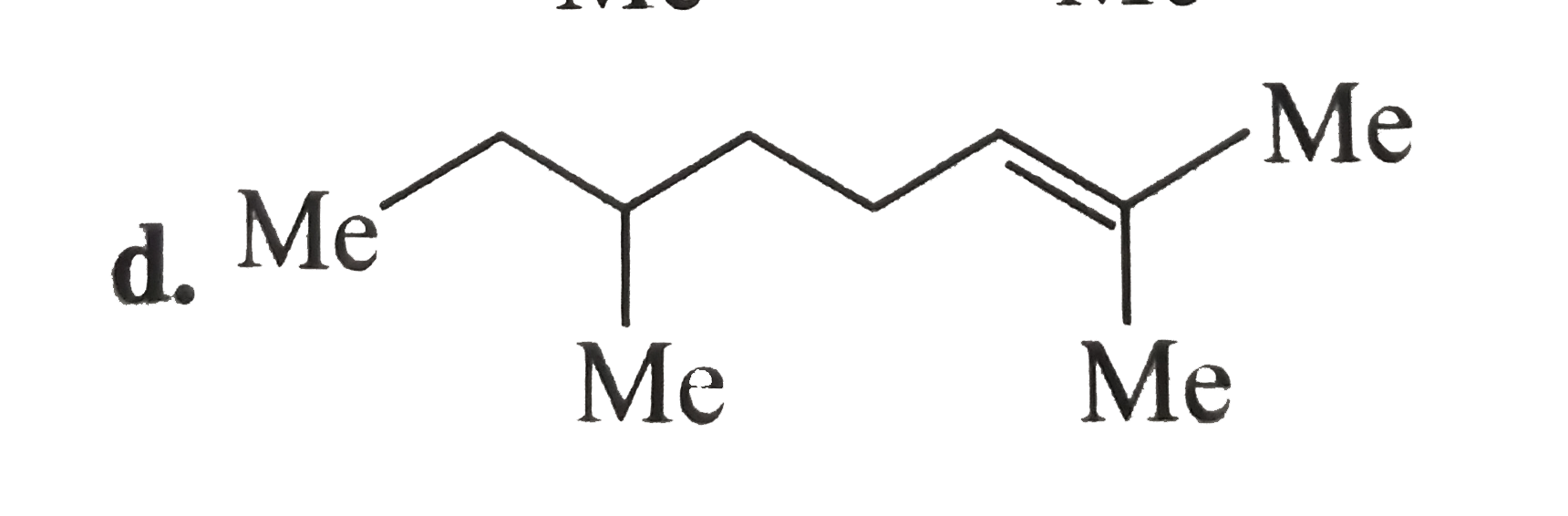
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ISOMERISM
CENGAGE CHEMISTRY ENGLISH|Exercise Multiple choice questions (Exercise)|38 VideosISOMERISM
CENGAGE CHEMISTRY ENGLISH|Exercise Single correct answer type|13 VideosISOMERISM
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise|19 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|28 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Chemical Equilibrium|73 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ISOMERISM-(Linked Comprehension Type)Exercise
- An organic compound (A) (C(10)H(20)) on reductive ozonolysis gives 2- ...
Text Solution
|
- An organic compound (A) (C(10)H(20)) on reductive ozonolysis gives 2- ...
Text Solution
|
- An organic compound (A) (C(10)H(20))on reductive ozonolysis gives 2- m...
Text Solution
|
- An organic compound (A) (C(10)H(20)) on reductive ozonolysis gives 2- ...
Text Solution
|
- An organic compound (A) (C(10)H(20))on reductive ozonolysis gives 2- m...
Text Solution
|
- An organic compound (A) (C(10)H(20)) on reductive ozonolysis gives 2- ...
Text Solution
|
- If the both compoundsd (A) and (B) are optically inactive, the possibl...
Text Solution
|
- If the both compoundsd (A) and (B) are optically inactive, the possibl...
Text Solution
|
- If the both compoundsd (A) and (B) are optically inactive, the possibl...
Text Solution
|
- How many isomaric dienes with a six-membered ring are possible of the ...
Text Solution
|
- In this paragraph, some statements are given based on isomerism. Read ...
Text Solution
|
- In this paragraph, some statements are given based on isomerism. Read ...
Text Solution
|
- In this paragraph, some statements are given based on isomerism. Read ...
Text Solution
|