Text Solution
Verified by Experts
Topper's Solved these Questions
THE P-BLOCK ELEMENTS
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section-J)|16 VideosTHE P-BLOCK ELEMENTS
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section-H)|10 VideosTHE D AND F-BLOCK ELEMENTS
AAKASH INSTITUTE ENGLISH|Exercise Assingment ( Section-J Aakash Challengers Questions )|10 VideosTHE S-BLOCK ELEMENTS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-J)|10 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-THE P-BLOCK ELEMENTS-Assignment Section-I)
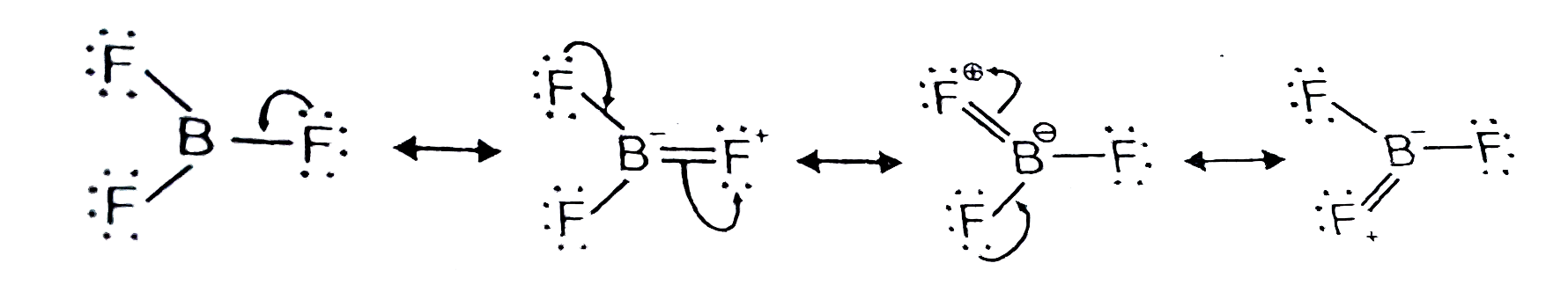
- (a) Explain why BF(3) exists whereas BH(3) does not. (b) Compare the...
Text Solution
|
- How is boron obtained from borax ? Give the chemical reactions involve...
Text Solution
|
- Boric acid can be titrated against sodium hydroxide using methyl orang...
Text Solution
|
- (a) BF(3) and BrF(3) molecule has different shapes. Expain. (b). C C...
Text Solution
|
- Although boric acid B(OH)(3), contains three hydroxyl groups yet it be...
Text Solution
|
- An aqueous solution containing one mole of HgI2 and two moles of NaI i...
Text Solution
|
- (a) On bolining a mineral (A) with Na2CO(3) solution, a white precipir...
Text Solution
|
- Complete the following .
Text Solution
|
- 'E' is same as graphite, identify (A) to (E)
Text Solution
|
- B(OH)(3) overset(HOCH(2)CH(2)OH)to?
Text Solution
|
- Indicate whether the following statements are true or false. Explain y...
Text Solution
|
- Write balanced equations for the reactions of the following compounds ...
Text Solution
|
- On gradual addition of KI solution to Bi(NO3)3 solution initially prod...
Text Solution
|
- An inorganic Lewis acid (X) shows the following reactions : (a) It f...
Text Solution
|
- Write balanced equation for reaction of XeF(4) with water. Also name t...
Text Solution
|
- Arrange in increasing order of extent of hydrolysis [C Cl(4),MgCl(2),A...
Text Solution
|