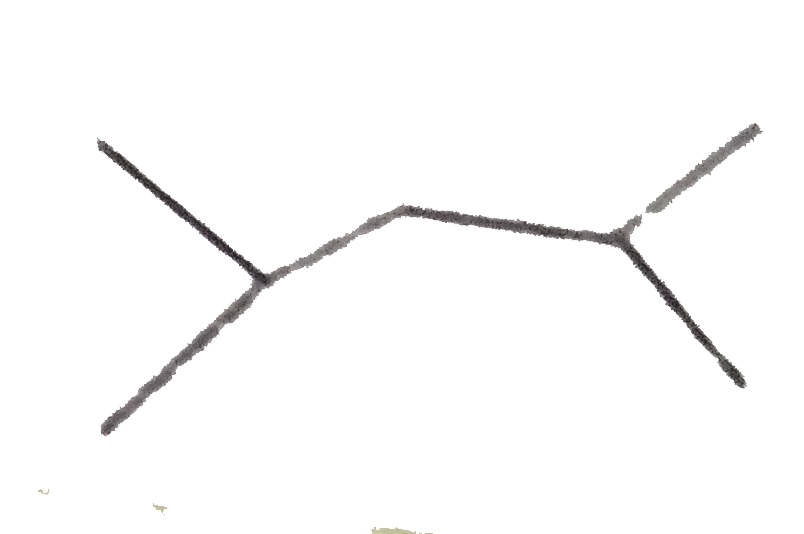
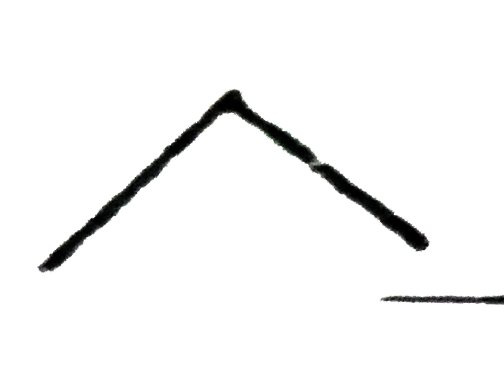
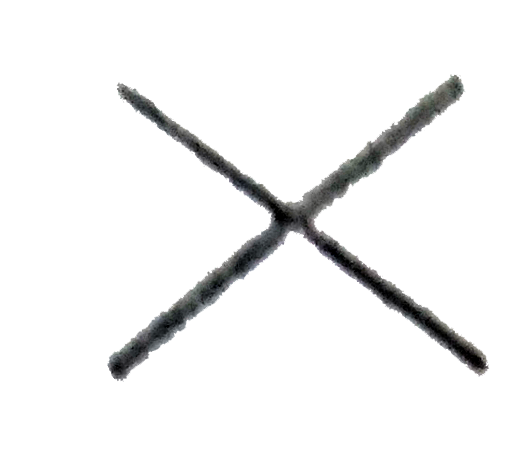
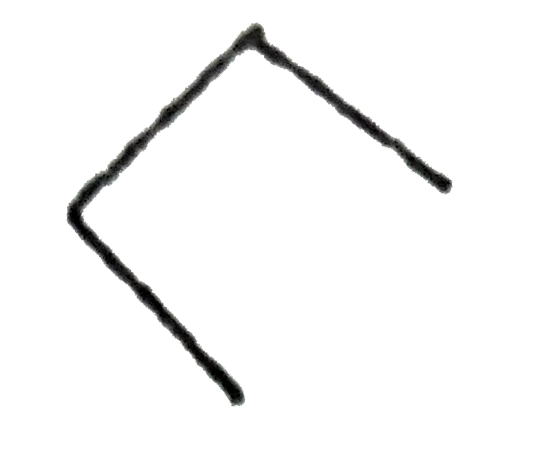
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
AAKASH INSTITUTE ENGLISH|Exercise SECTION-D LINKED COMPREHENSION TYPE QUESTIONS|7 VideosHYDROCARBONS
AAKASH INSTITUTE ENGLISH|Exercise SECTION-E ASSERTION-REASON TYPE QUESTIONS:|9 VideosHYDROCARBONS
AAKASH INSTITUTE ENGLISH|Exercise SECTION-B OBJECTIVE TYPE QUESTIONS (ONE OPTION IS CORRECT)|25 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT SECTION -D|15 VideosHYDROGEN
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION - D) (Assertion-Reason Type question)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-HYDROCARBONS-SECTION-C OBJECTIVE TYPE QUESTIONS (MORE THAN ONE OPTIONS ARE CORRECT)
- Which of the folloiwng alkanes will give more than one monochloro prod...
Text Solution
|
- In which of the following cases product will contain more number of ca...
Text Solution
|
- Which of the following name reaction is used to prepare alkane contain...
Text Solution
|
- The photochemical chlorination of paraffins occurs by a free radical m...
Text Solution
|
- The concentration aqueous solution of potassium salts of acetic acid a...
Text Solution
|
- Predict the product of tiven reaction
Text Solution
|
- Which of the following reagents on reaction with acetylene yeild same ...
Text Solution
|
- In which of the following case product case product of oxidative and r...
Text Solution
|
- Structures of sigma-complex formed during nitration of Anisole would b...
Text Solution
|
- Which of the following reagents can be used to prepare 2-butyne by sim...
Text Solution
|
- Friedel-Crafts alkylation is expected to proceed through carbocationic...
Text Solution
|
- Which of the following statements is/are correct regarding catalytic h...
Text Solution
|