Text Solution
Verified by Experts
Topper's Solved these Questions
HYDROCARBONS
AAKASH INSTITUTE ENGLISH|Exercise Try yourself|78 VideosHYDROCARBONS
AAKASH INSTITUTE ENGLISH|Exercise Exercise|33 VideosHYDROCARBONS
AAKASH INSTITUTE ENGLISH|Exercise SECTION-I SUBJECTIVE QUESTIONS|9 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT SECTION -D|15 VideosHYDROGEN
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION - D) (Assertion-Reason Type question)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-HYDROCARBONS-SECTION-J AAKASH CHALLENGERS QUESTIONS
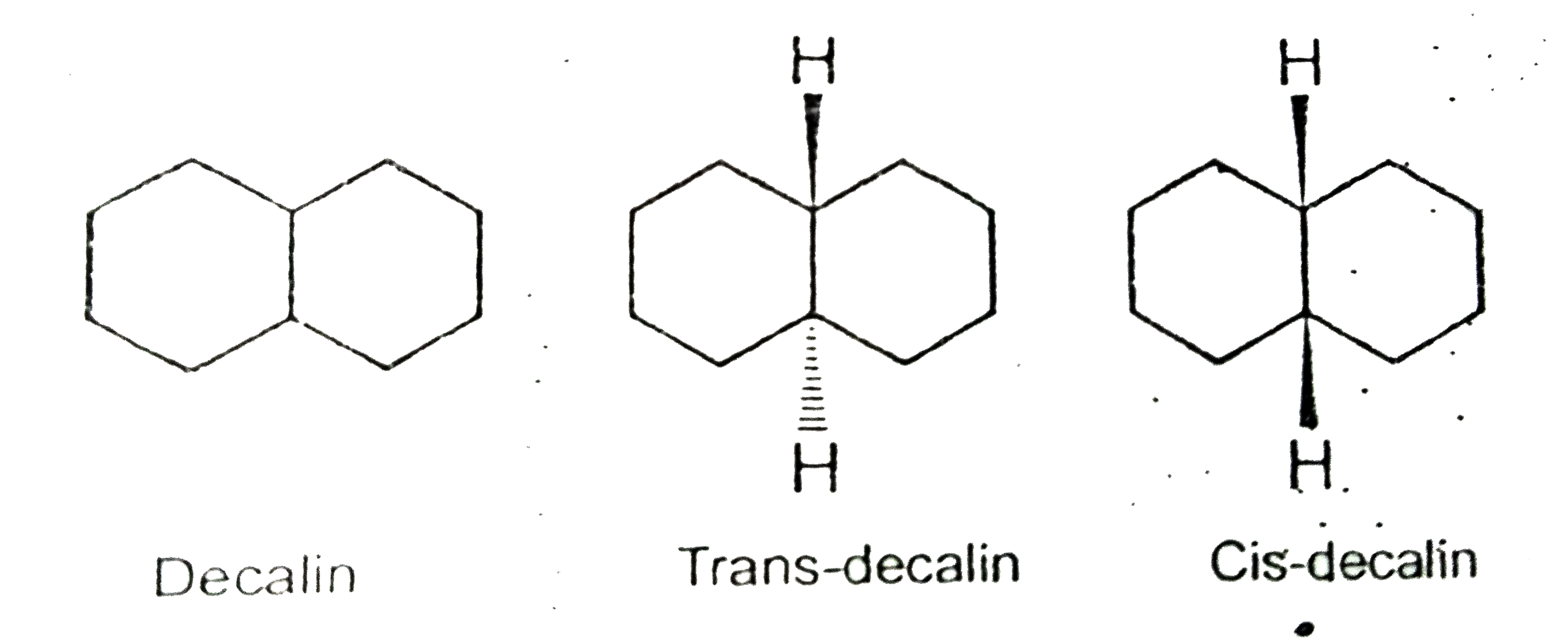
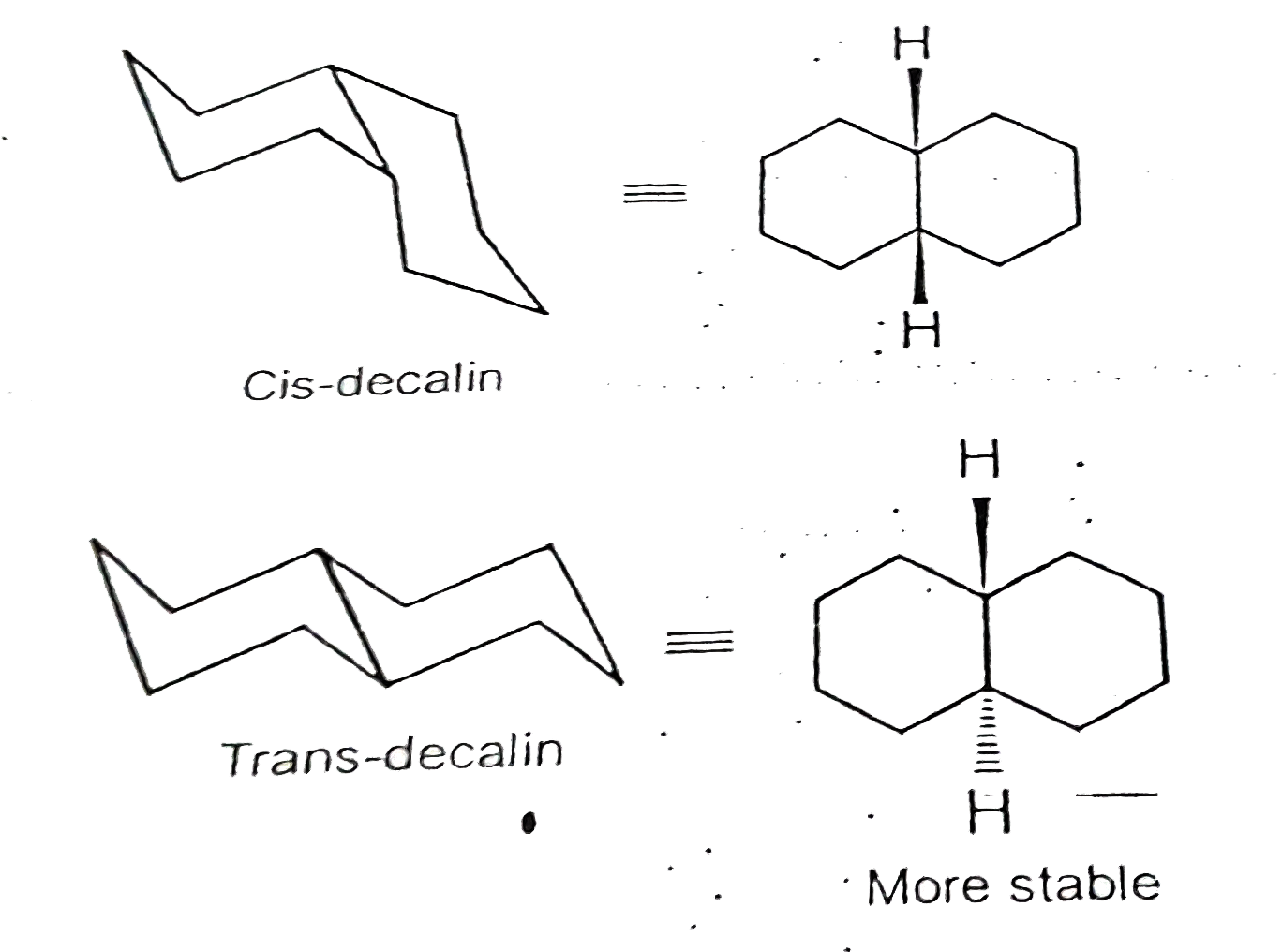
- Decalin is an example of fused bicyclic systems where two six membered...
Text Solution
|
- Which double bond in the given molecule is most reactive towards an el...
Text Solution
|
- What product would be obtained from the reaction of cyclopropane with ...
Text Solution
|
- Assuming that no rearrangement is taking place, then how many hydrocar...
Text Solution
|
- Arrangte the following hydrocarbons in the increasing order of enthalp...
Text Solution
|