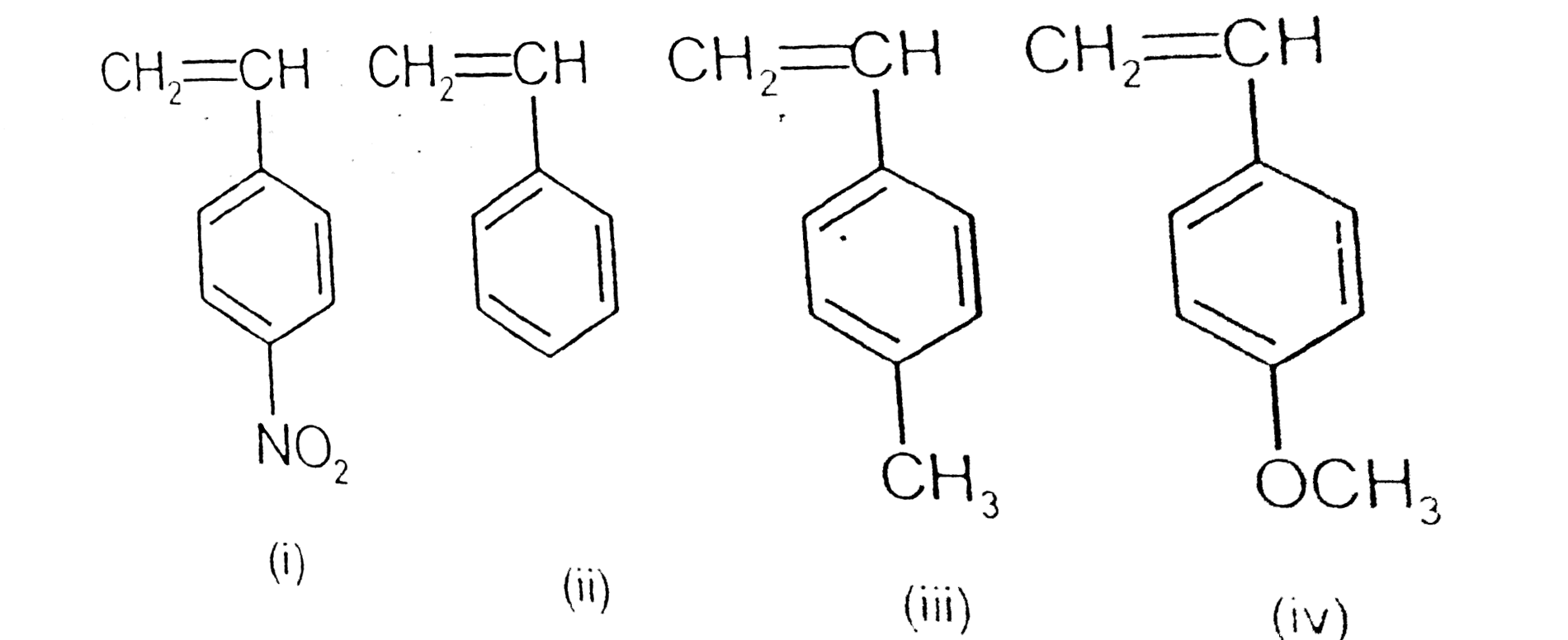
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
POLYMERS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT Section- E (Assertion-Reason Type Questions )|3 VideosPOLYMERS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT Section-F (Matrix match Type Questions )|6 VideosPOLYMERS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT Section -C (Objective type Questions (More one option is correct ))|8 VideosORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES
AAKASH INSTITUTE ENGLISH|Exercise TRY YOURSELF|28 VideosPRINCIPLES OF QUALITATIVE ANALYSIS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION H)|9 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-POLYMERS-ASSIGNMENT Section-D (Linked Comprehension Type Questions )
- In cationic polymerizalion, the initiator is an electrophile that adds...
Text Solution
|
- In cationic polymerizalion, the initiator is an electrophile that adds...
Text Solution
|
- In cationic polymerizalion, the initiator is an electrophile that adds...
Text Solution
|
- Addition polymers are those in which monomers are converting into poly...
Text Solution
|
- Addition polymers are those in which monomers are converting into poly...
Text Solution
|
- The condensation polymer are those which gives out some simple molecul...
Text Solution
|
- The condensation polymer are those which gives out some simple molecul...
Text Solution
|