A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES, KETONES AND CARBOXYLIC ACIDS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION -B OBJECTIVE TYPES QUESTIONS (ONE OPTION IS CORRECT)|24 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION -C Objective type questions more than one options are correct)|11 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
AAKASH INSTITUTE ENGLISH|Exercise Illustrations|3 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH INSTITUTE ENGLISH|Exercise Try Yourself|5 VideosAMINES
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section -J (Aakash Challengers Questions)|9 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-ALDEHYDES, KETONES AND CARBOXYLIC ACIDS -Assignment (Section A Competition Level Questions)
- Which of the following is mixed ketone?
Text Solution
|
- The reaction of acetaldehyde with HCN followed by hydrolysis gives a p...
Text Solution
|
- Which factors will increase the reactivity of group? (i) Presence of...
Text Solution
|
- Which of the following is optically active?
Text Solution
|
- identify the wrong statement from the following:
Text Solution
|
- The general formula of C(n)H(2n)O(2) could be for open chain
Text Solution
|
- The IUPAC name of the phthalic acid-
Text Solution
|
- In the following reaction, product (P ) is R-overset(O)overset("||")...
Text Solution
|
- Dry distillation of calcium acetate gives :
Text Solution
|
- CH(3)-CH(2)-C -=CH overset( R)underset(H(3)O^(oplus))to Butanone, R is
Text Solution
|
- Which of the following pathways produces 2-hexanone? (i) 1-Hexyne is...
Text Solution
|
- An alkene of molecular formula C(9) H(18) on ozonolysis gives 2.2 dime...
Text Solution
|
- (CH(3))(2)COoverset(NaCN)underset(HCI)rarr(A)underset(Delta)overset(H(...
Text Solution
|
- A liquid was mixed with ethanol and a drop of concentrated H(2) SO(4) ...
Text Solution
|
- The major product obtained on interaction of phenol with sodium hydrox...
Text Solution
|
- Which of the following does not give benzoic acid on hydrolysis?
Text Solution
|
- The compound x is
Text Solution
|
- Acetic acid is obtained when
Text Solution
|
- When m-chlorobenzaldehyde is treated with 50% KOH solution, the produc...
Text Solution
|
- Aldol condensation will not be observed in
Text Solution
|
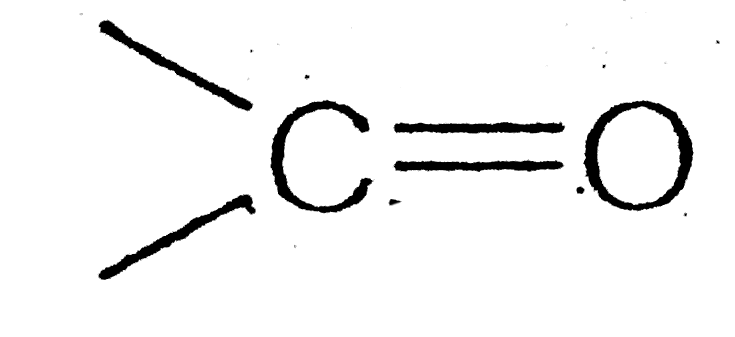 group?
group?