A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
KINETIC THEORY
AAKASH INSTITUTE ENGLISH|Exercise EXERCISE (ASSIGNMENT) SECTION - B Objective Type Questions|30 VideosKINETIC THEORY
AAKASH INSTITUTE ENGLISH|Exercise EXERCISE (ASSIGNMENT) SECTION - C Previous Years Questions|21 VideosKINETIC THEORY
AAKASH INSTITUTE ENGLISH|Exercise EXERCISE|10 VideosGRAVITATION
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT SECTION - D (ASSERTION-REASON TYPE QUESTIONS)|15 VideosLAWS OF MOTION
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION-D) (Assertion-Reason Type Questions)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-KINETIC THEORY-EXERCISE (ASSIGNMENT) SECTION - A Objective Type Questions
- Which of the following methods will enable the volume of an ideal gas ...
Text Solution
|
- A container has N molecules at absolute temperature T. If the number o...
Text Solution
|
- During an experiment, an ideal gas is found to obey an additional law ...
Text Solution
|
- At what temperature, pressure remaining constant will the r.m.s. speed...
Text Solution
|
- Two thermally insulated vessels (1) and (2) are filled with air at tem...
Text Solution
|
- The average speed of gas molecules is v at pressure P, If by keeping t...
Text Solution
|
- Four molecules of gas have speeds 1,2,3 and 4 km//s.The value of the r...
Text Solution
|
- The rms speed of the molecule of enclosed gas is v. What will be the r...
Text Solution
|
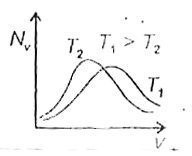
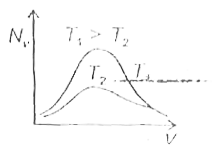
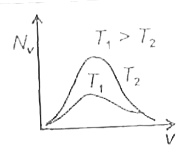
- The effect of temperature on Maxwell's speed distribution is correctly...
Text Solution
|
- Select the incorrect statement about Maxwell's speed distribution.
Text Solution
|
- The ratio of number of collisions per second at the walls of container...
Text Solution
|
- An ant is moving on a plane horizontal surface. The number of degrees ...
Text Solution
|
- If a gas has n degrees of freedom ratio of specific heats of gas is
Text Solution
|
- The molar specific heat at constant volume, for a non linear triatomic...
Text Solution
|
- A mixture of ideal gases has 2 moles of He, 4 moles of oxygen and 1 mo...
Text Solution
|
- E(0) and E(n) respectively represent the average kinetic energy of a ...
Text Solution
|
- 14 g of CO at 27^@ C is mixed with 16'g of O2 at 47^@ C. The temperatu...
Text Solution
|
- When one mole of monoatomic gas is mixed with one mole of triatomic ga...
Text Solution
|
- A box of negligible mass containing 2 moles of an ideal gas of molar m...
Text Solution
|
- On increasing number density for a gas in a vessel, mean free path of ...
Text Solution
|