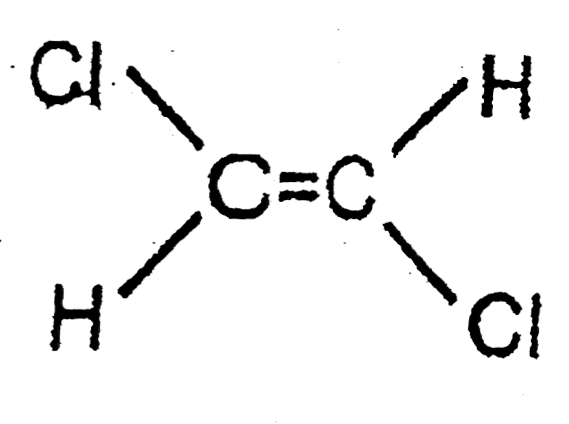
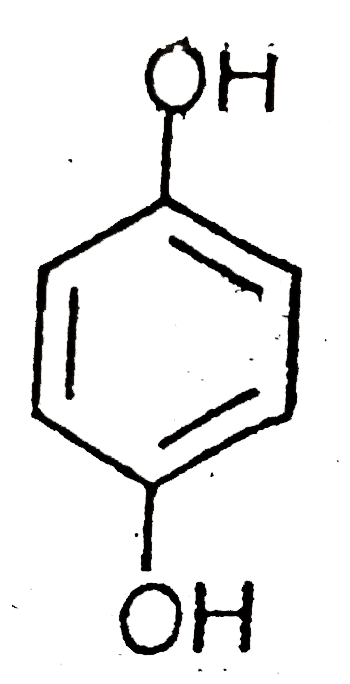
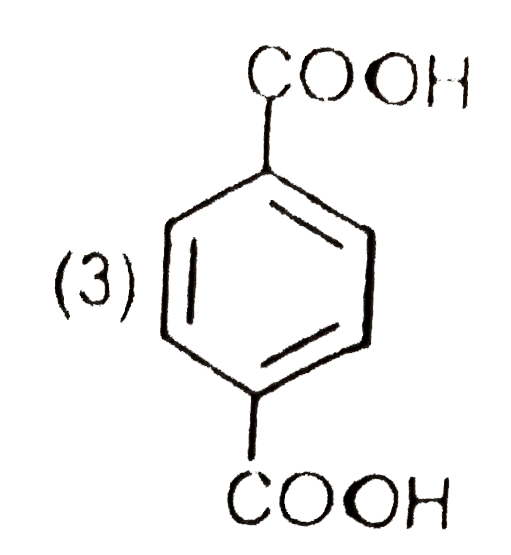
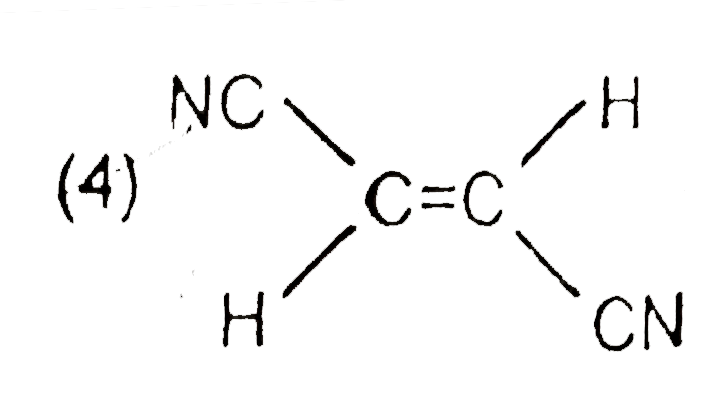
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following is/are nonpolar (mu = 0) ?
Text Solution
|
- Which of the following has the maximum solubility in a given nonpolar ...
Text Solution
|
- Which of the following compounds of xenon is nonpolar?
Text Solution
|
- Which of the following is/are nonpolar (mu = 0) ?
Text Solution
|
- Out of the following which reaction give nonpolar product?
Text Solution
|
- Which of the following compound has mu= 0 ?
Text Solution
|
- In which of the following both are nonpolar -
Text Solution
|
- The amino acid which has a nonpolar side chain is
Text Solution
|
- Which among the following solids is a nonpolar solid?
Text Solution
|