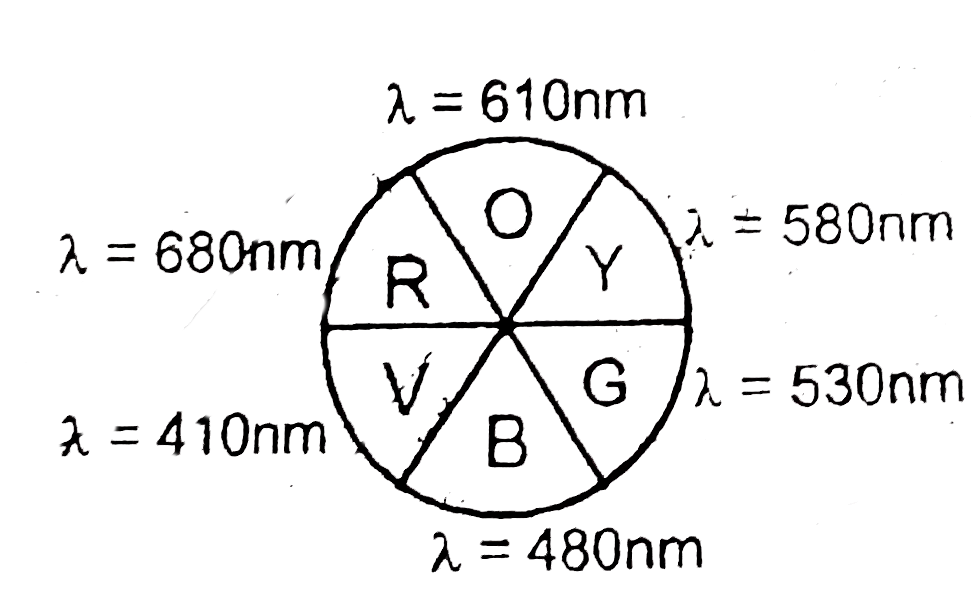
A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Complex [FeL(6)]^(2+) is yellow in colour then expected magnetic momen...
Text Solution
|
- The complex Ca(2) [M (CN)](6) has spin only magnetic moment 2.83 BM an...
Text Solution
|
- The complexes that have a magnetic moment of 1.73 BM is
Text Solution
|
- the pair of metal ions that can give a spin - only magnetic moment o...
Text Solution
|
- Which of the complexes has the magnetic moment of 3.87 BM ?
Text Solution
|
- The magnetic moment of [MnX(4)]^(2-) is 5.9 BM. The geometry of the co...
Text Solution
|
- The magnetic moment of a complex ion is 1.73 BM. The complex ion is .
Text Solution
|
- The magnetic moment of a complex ion is 2.83 BM. The complex ion is :
Text Solution
|
- In which of the following complex(s) spin only magnetic moment is inde...
Text Solution
|