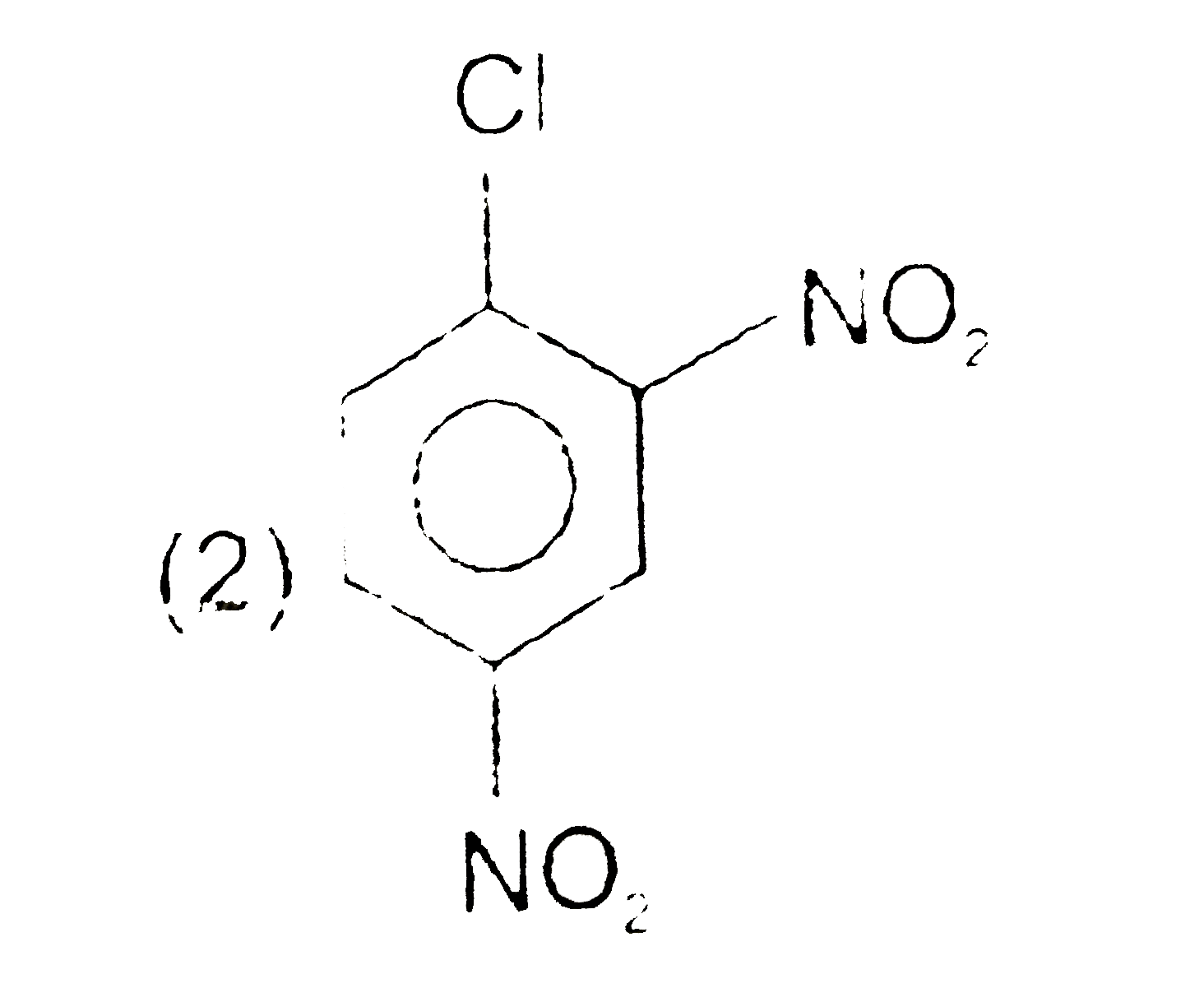
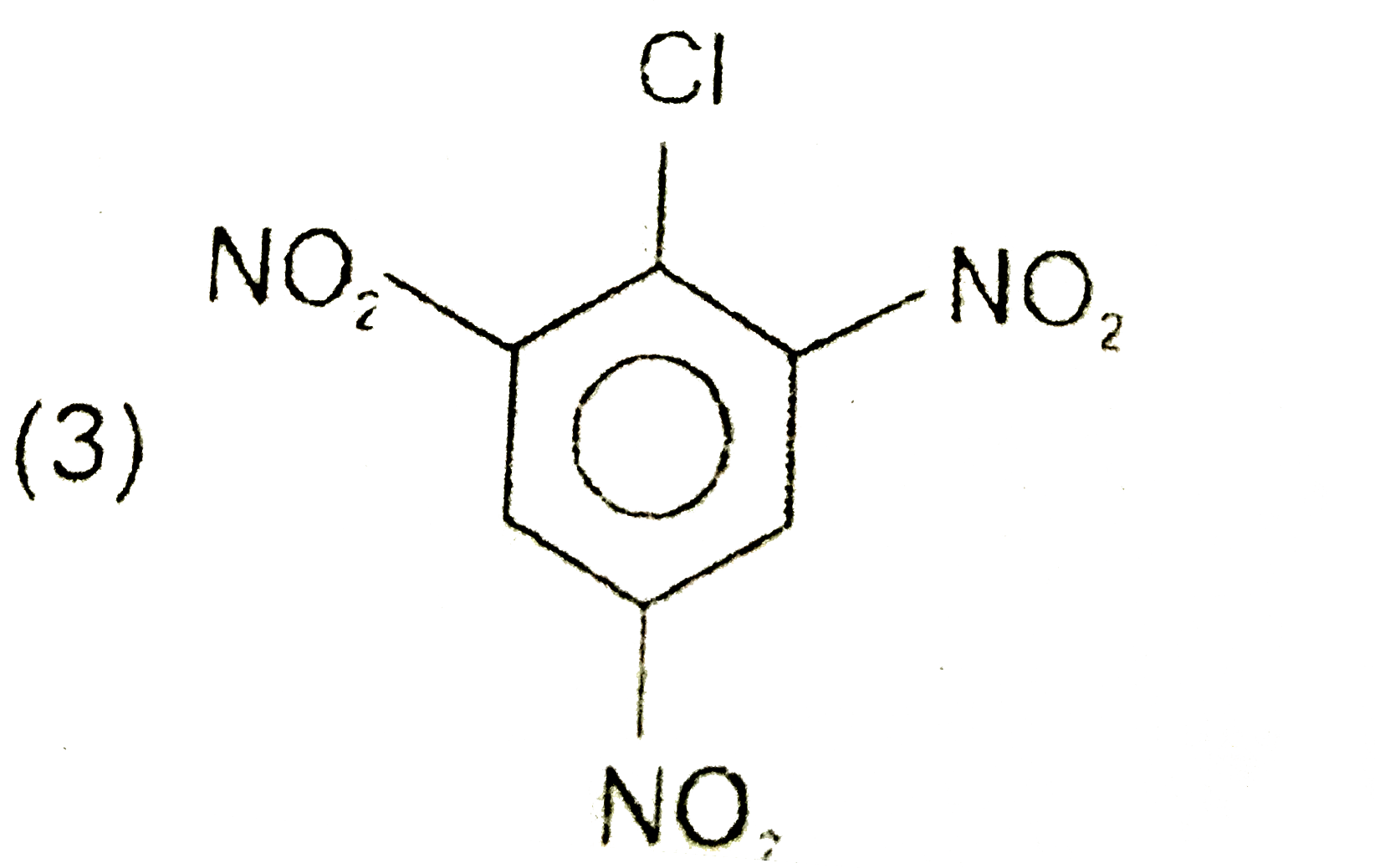
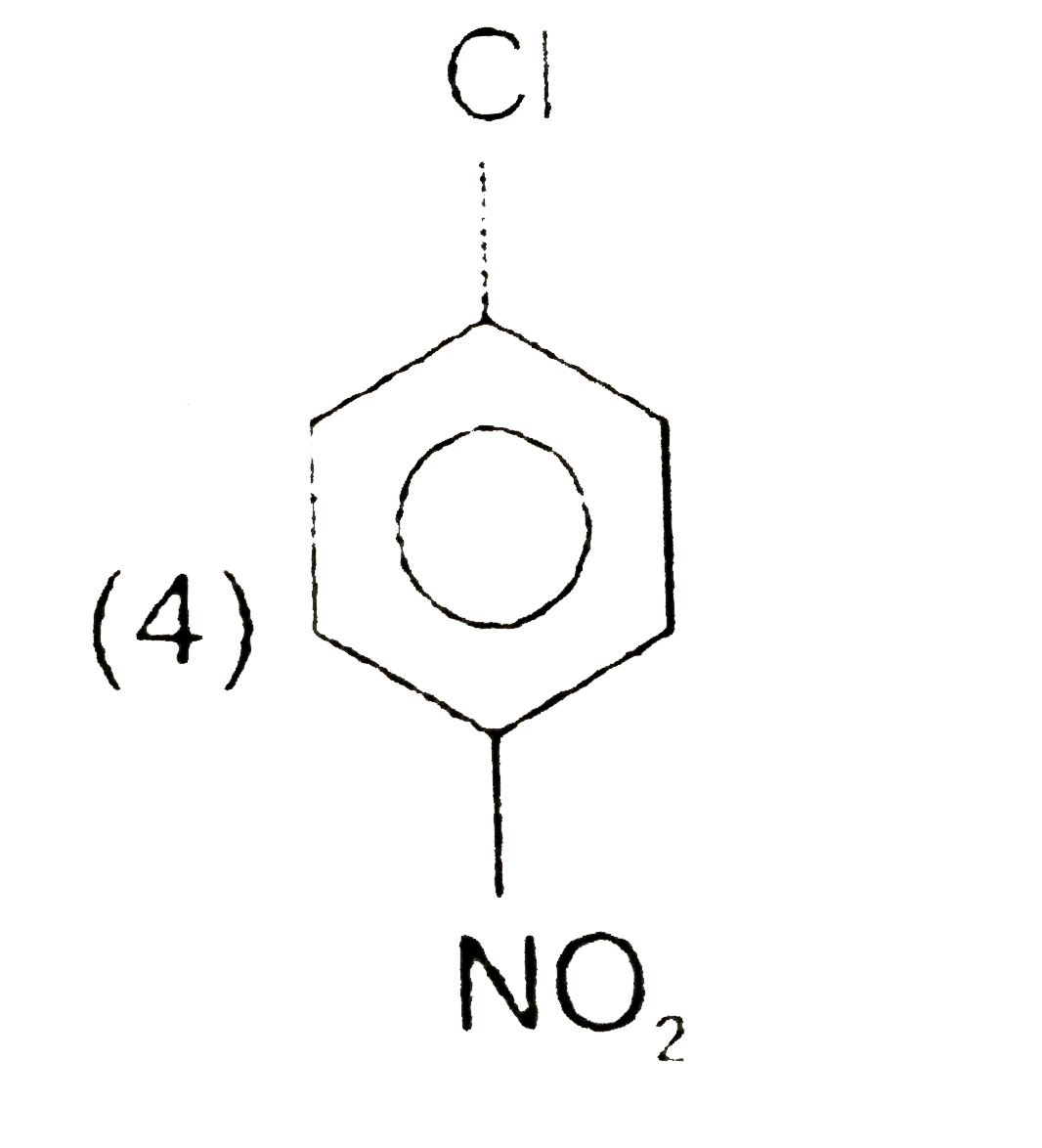
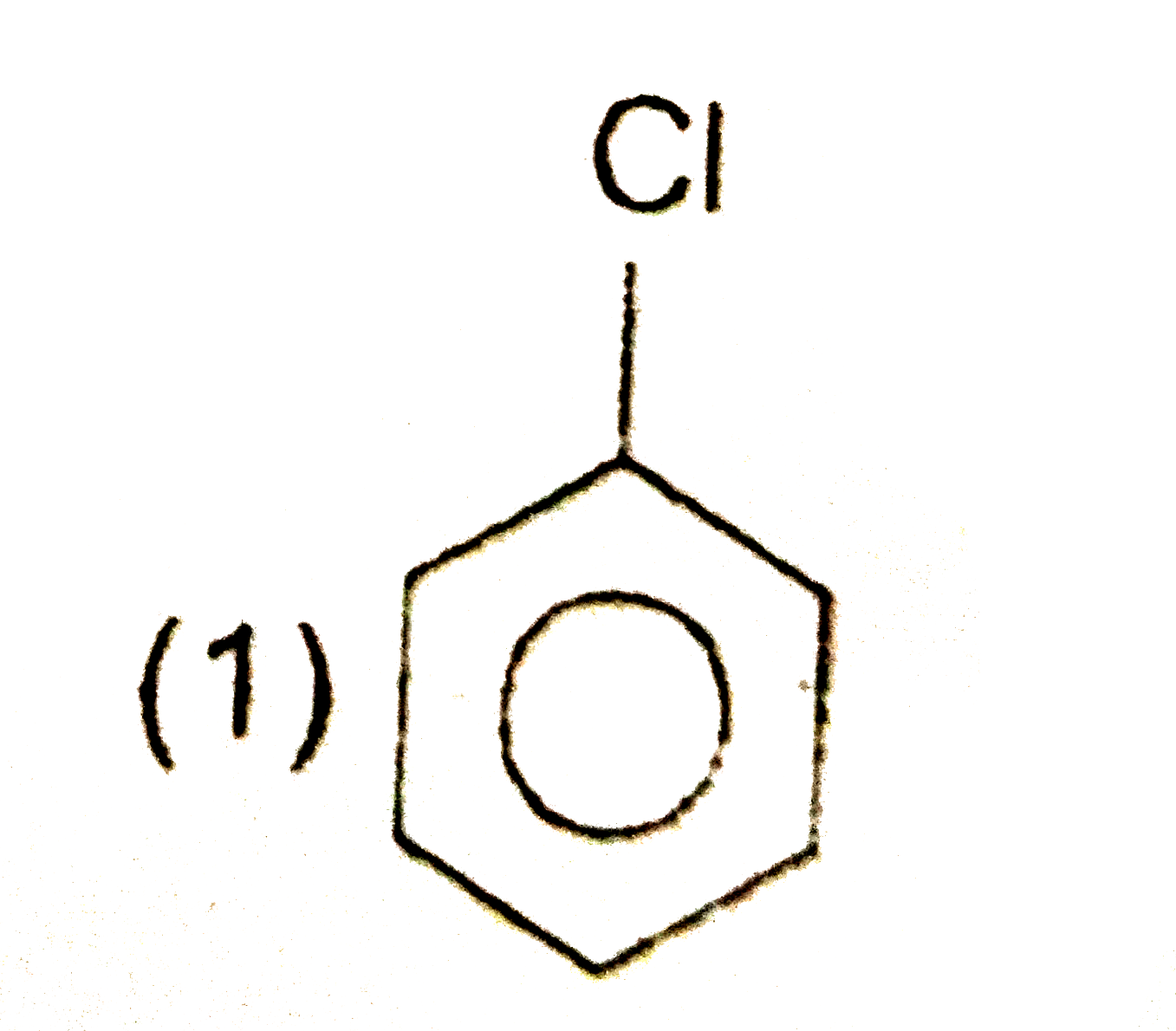A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following compound gives fastest S(N)2Ar reaction?
Text Solution
|
- Which of the following compound gives fastest S(N)2Ar reaction?
Text Solution
|
- The rate of S(N^(1)) reaction is fastest with :
Text Solution
|
- Which of the following compounds will not give S(N^(2)) reaction?
Text Solution
|
- Which of the following compounds will give S(N^(1)) reaction?
Text Solution
|
- Which of the following compounds will give racemic mixture by S(N^(1))...
Text Solution
|
- Which of the following alkyl halides undergoes the fastest S(N^(1)) re...
Text Solution
|
- Which will undergo S(N^(2)) reaction fastest amont the following halog...
Text Solution
|
- S(N)2 reaction will be fastest in:
Text Solution
|