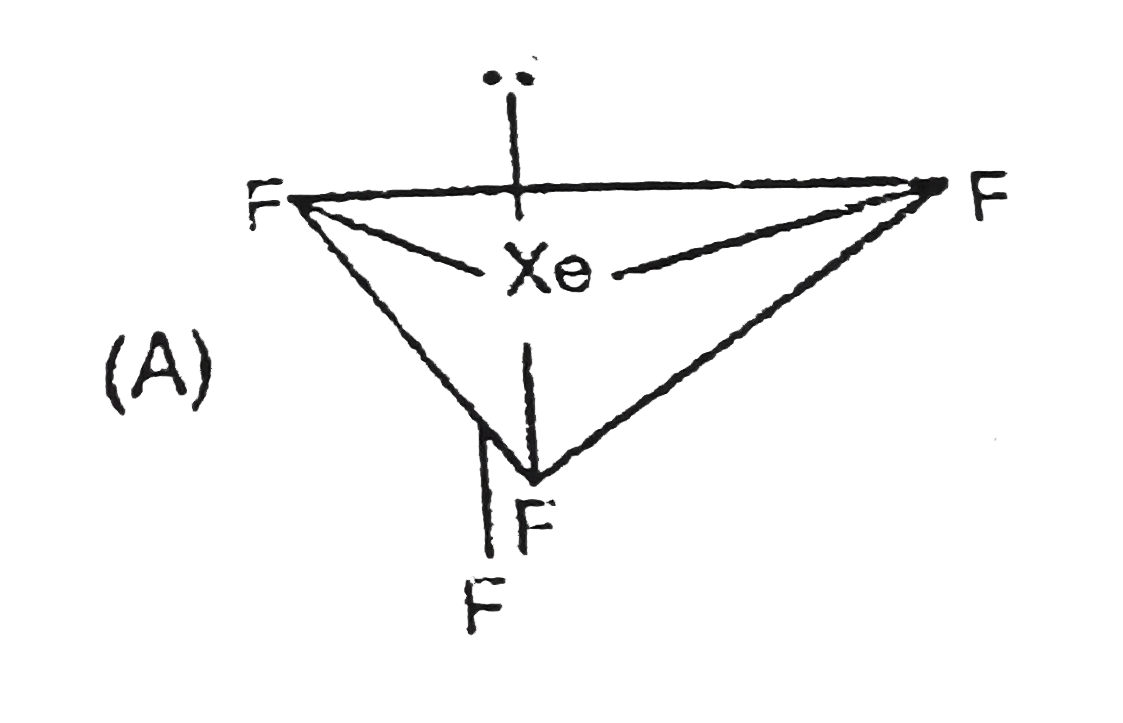
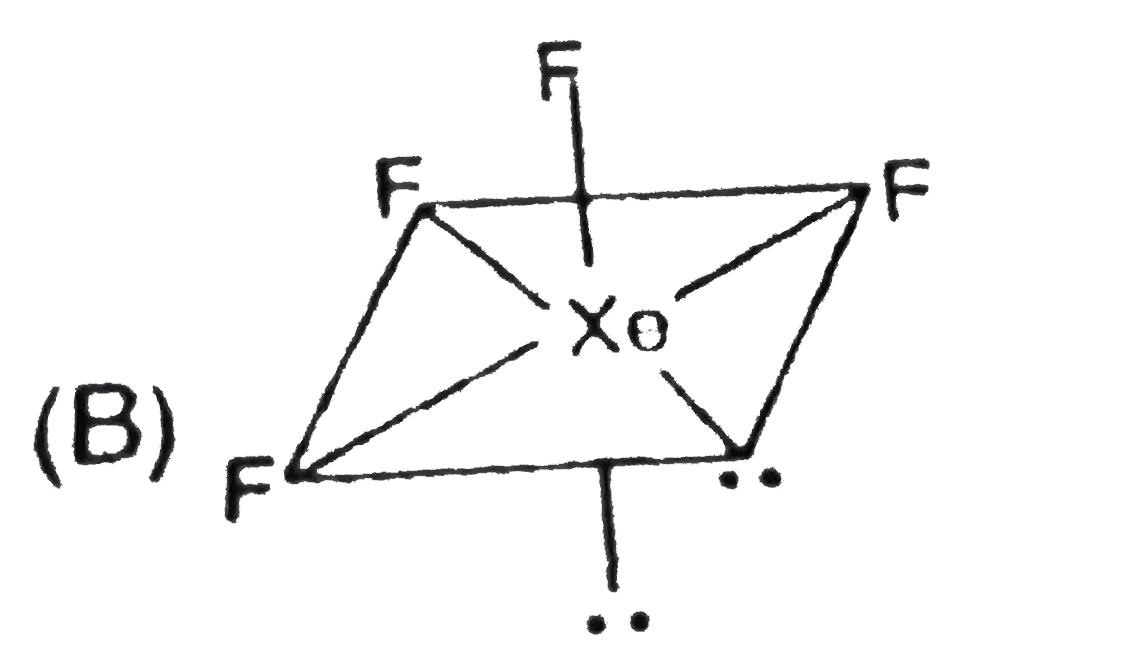
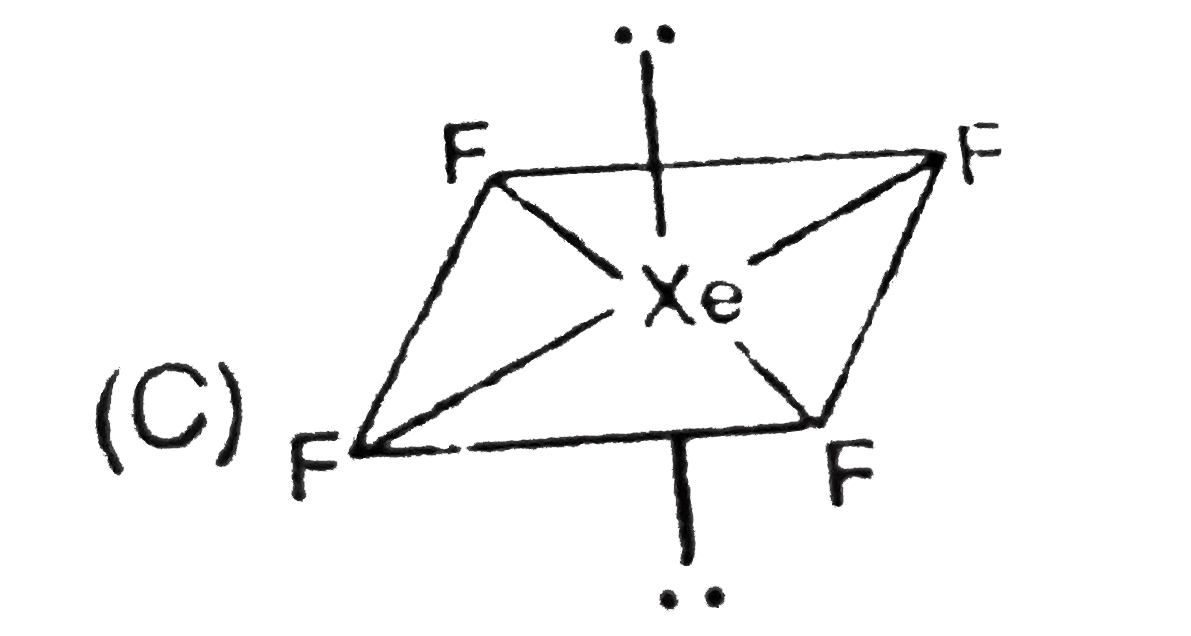
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- A chemical bond between two atoms is:
Text Solution
|
- Define isotopes and give an example
Text Solution
|
- Draw the structure of XeF4 molecule.
Text Solution
|
- Match the following and choose the correct option given below (a) N(...
Text Solution
|
- During the bond formation, energy is absorbed. (T/F)
Text Solution
|
- According to molecular orbital theory which of the following is correc...
Text Solution
|
- 80 mL of pure phosphine is decomposed to produce vapours of phosphorus...
Text Solution
|
- 140 mL of pure phosphine is decomposed to produce vapours of phosphoru...
Text Solution
|
- A polythene bag 3 litre capacity is partially filled by 1 liter of Hel...
Text Solution
|
- Consider the following statements and arrange in the order of true/fal...
Text Solution
|
- Select the correct statement(s) with respect to the p pi-dpi dative bo...
Text Solution
|
- A flask contain 88g of CO2 gas. Calculate how many moles of CO2 gas do...
Text Solution
|
- A flask contain 44g of CO2 gas. Calculate how many moles of CO2 gas do...
Text Solution
|
- A polythene bag 3 litre capacity is partially filled by 1 liter of Hel...
Text Solution
|
- Select that pairs in which bond angle of first member is higher than s...
Text Solution
|
- Which charge for the N2 molecule would give a bond order of 2.5 ?
Text Solution
|
- 130 mL of pure phosphine is decomposed to produce vapours of phosphoru...
Text Solution
|
- 180 mL of pure phosphine is decomposed to produce vapours of phosphoru...
Text Solution
|
- A flask contain 132g of CO2 gas. Calculate how many moles of CO2 gas d...
Text Solution
|
- A flask contain 13.2g of CO2 gas. Calculate how many moles of CO2 gas ...
Text Solution
|