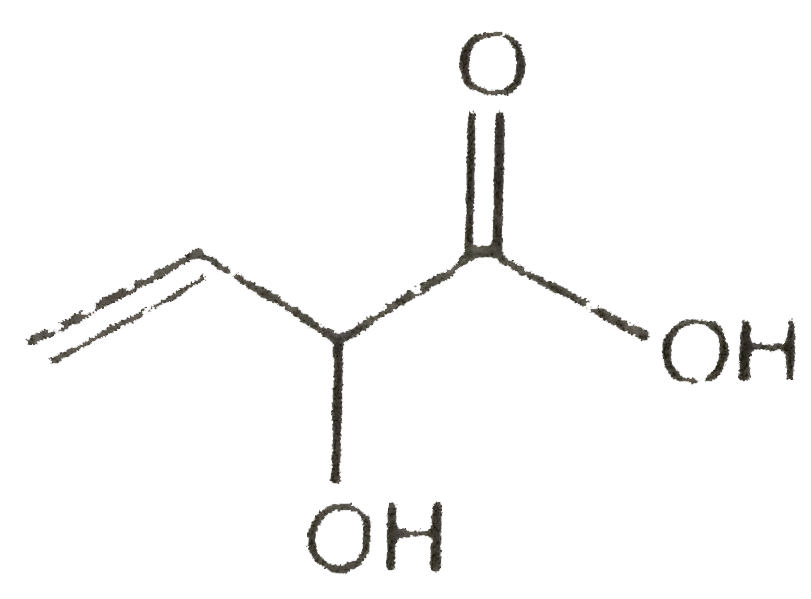
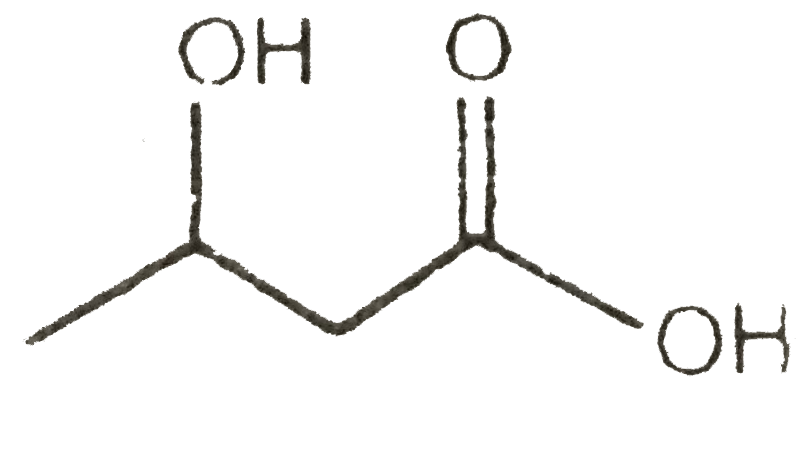
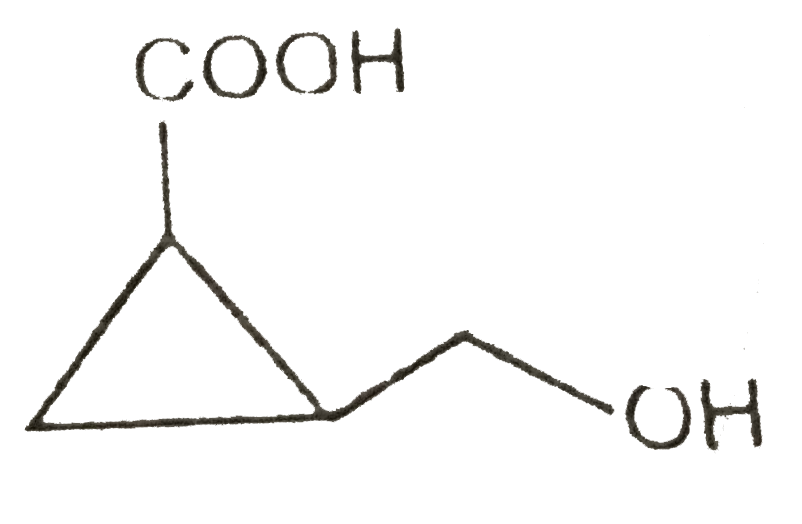
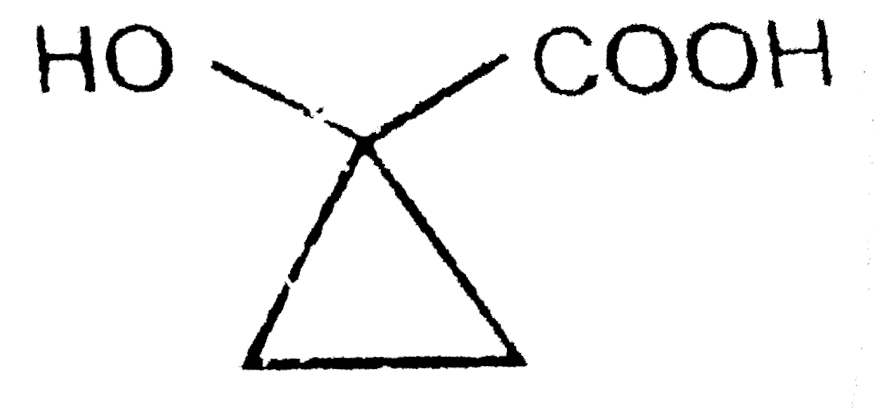A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
IUPAC NOMENCLATURE & STRUCTURAL ISOMERISM
RESONANCE ENGLISH|Exercise Exercise-3 Part-1|9 VideosIUPAC NOMENCLATURE & STRUCTURAL ISOMERISM
RESONANCE ENGLISH|Exercise Exercise-3 Part-2|8 VideosIUPAC NOMENCLATURE & STRUCTURAL ISOMERISM
RESONANCE ENGLISH|Exercise Exercise-2 Part-3|10 VideosIONIC EQUILIBRIUM
RESONANCE ENGLISH|Exercise partIII one or more than one options correct type|10 VideosMETALLURGY
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Alkyl Halide, Alcohol,Phenol,Ether)|17 Videos
Similar Questions
Explore conceptually related problems