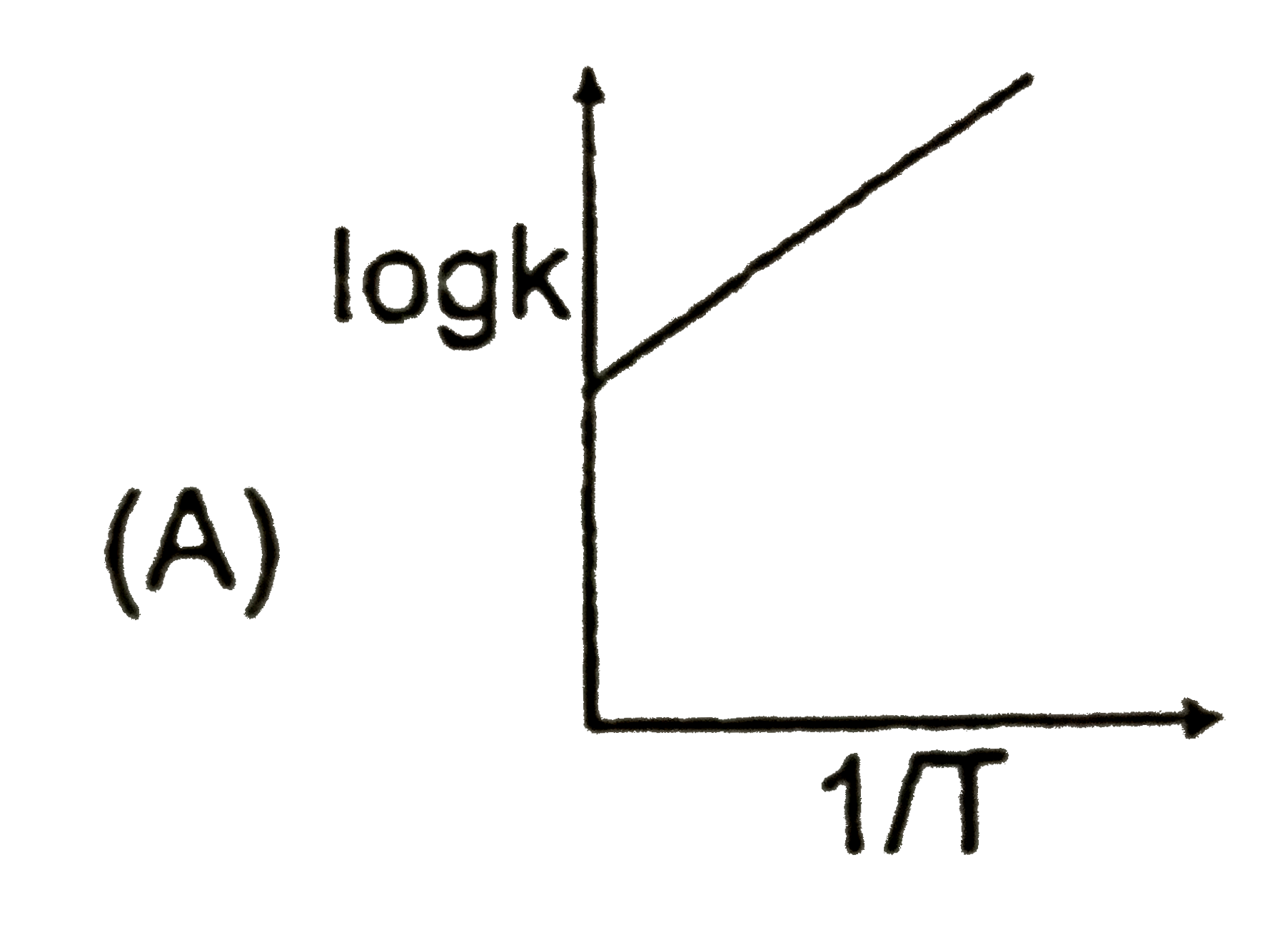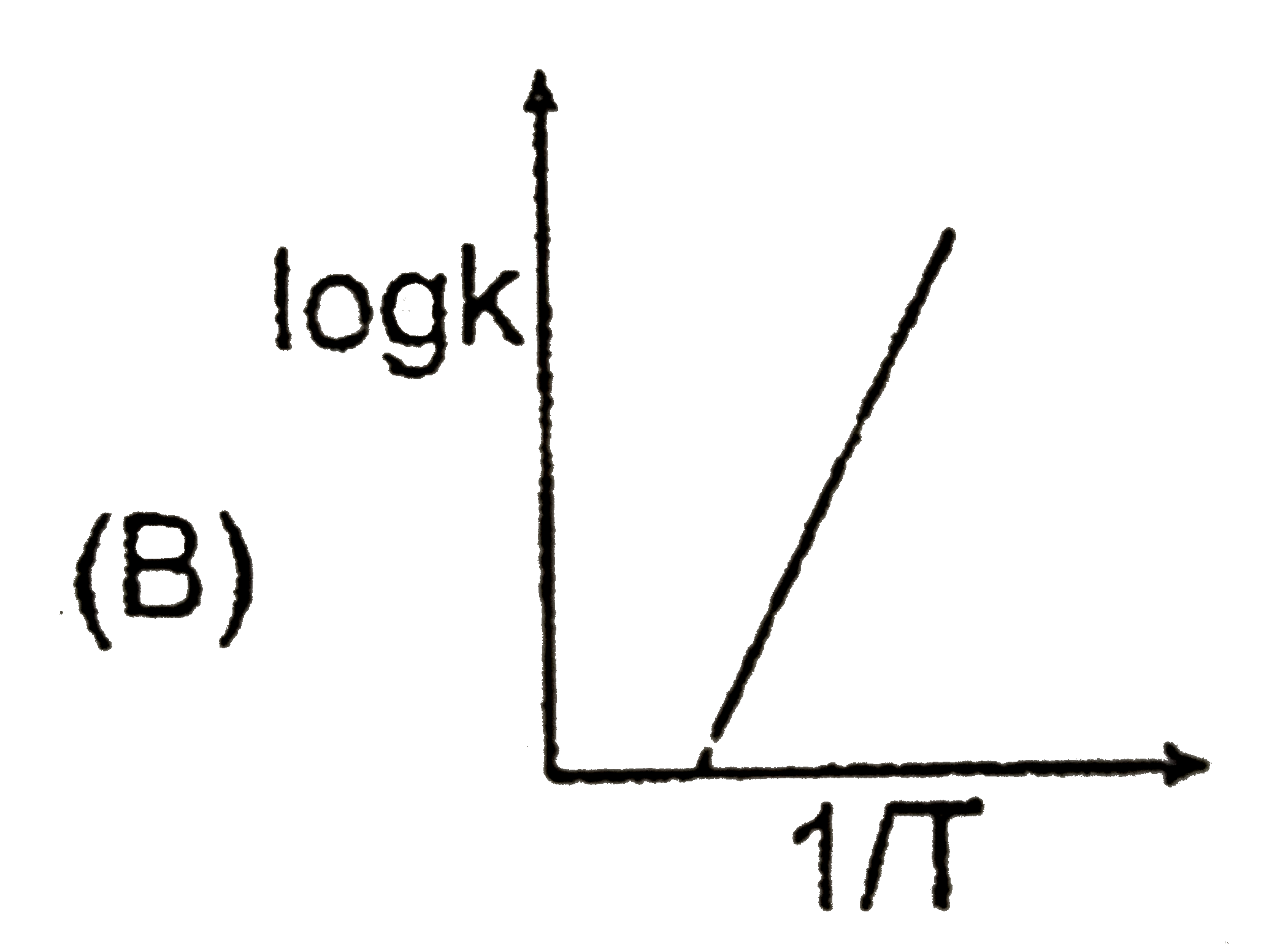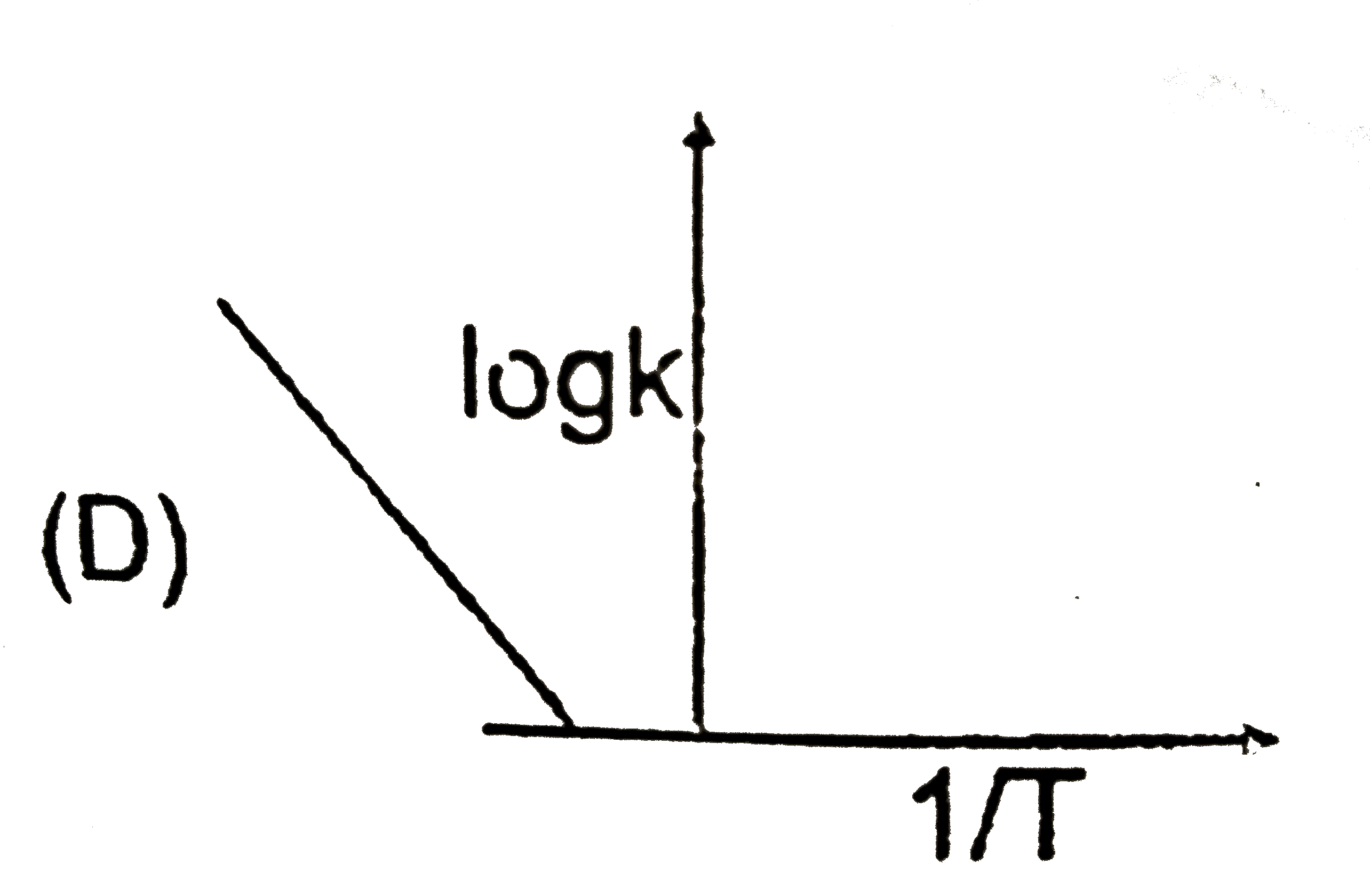A
B
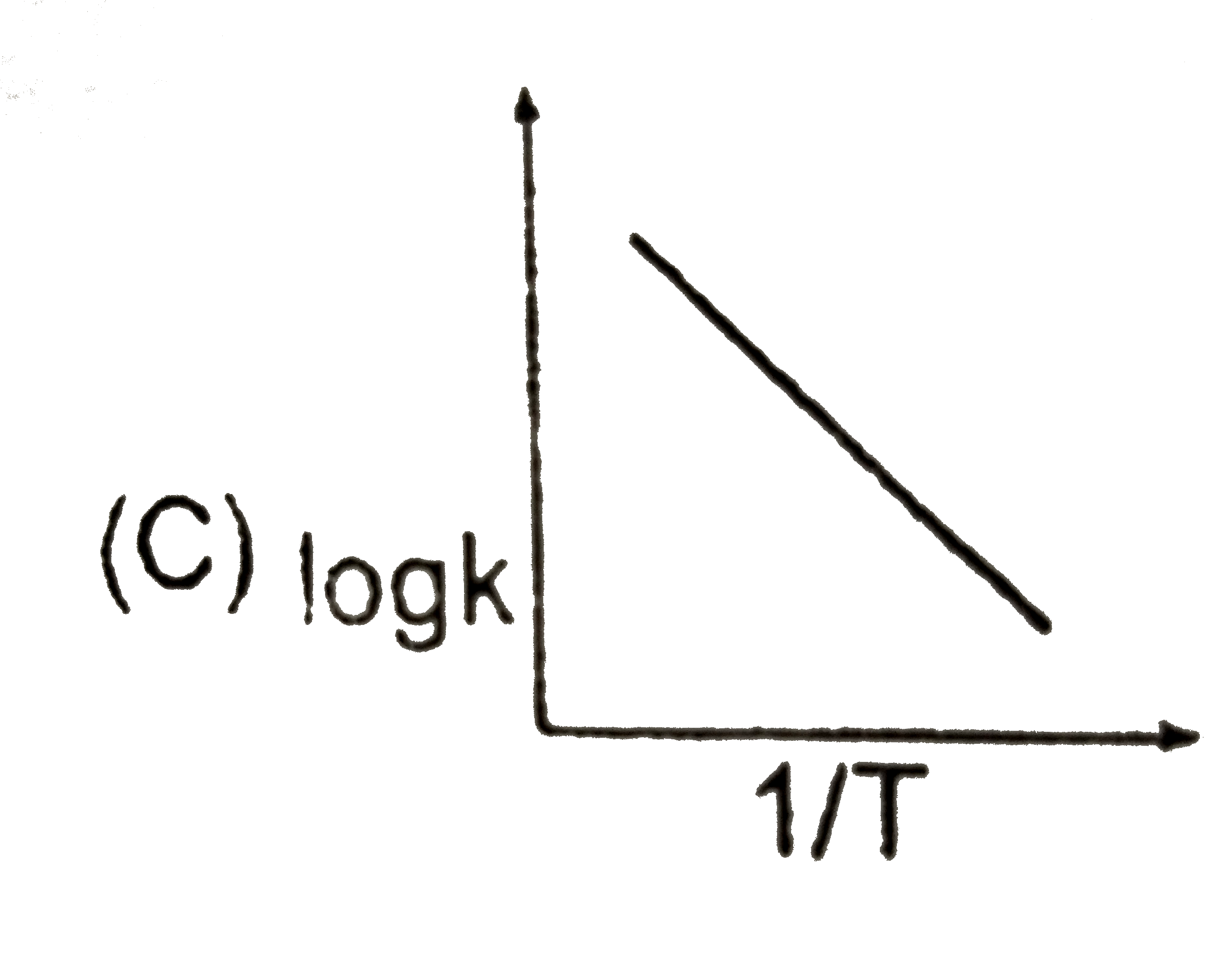
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
RESONANCE ENGLISH|Exercise Exercise-3 (Part-1)|6 VideosCHEMICAL EQUILIBRIUM
RESONANCE ENGLISH|Exercise Exercise-3 (Part-2)|17 VideosCHEMICAL EQUILIBRIUM
RESONANCE ENGLISH|Exercise Exercise-2 (Part-3)|33 VideosCHEMICAL BONDING
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Fundamental Concept )|6 VideosD & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS
RESONANCE ENGLISH|Exercise Match the column|1 Videos
RESONANCE ENGLISH-CHEMICAL EQUILIBRIUM-Exercise-2 (Part-4)
- Le Chatelier's Principle If a system at equilibrium is subjected to ...
Text Solution
|
- Le Chatelier's Principle If a system at equilibrium is subjected to ...
Text Solution
|
- For the reaction N(2)(g)+O(2)(g)hArr2NO(g) If pressure id increase...
Text Solution
|
- Effect of temperature on the equilibrium process analysed by using the...
Text Solution
|
- Effect of temperature on the equilibrium process analysed by using the...
Text Solution
|
- Effect of temperature on the equilibrium process analysed by using the...
Text Solution
|