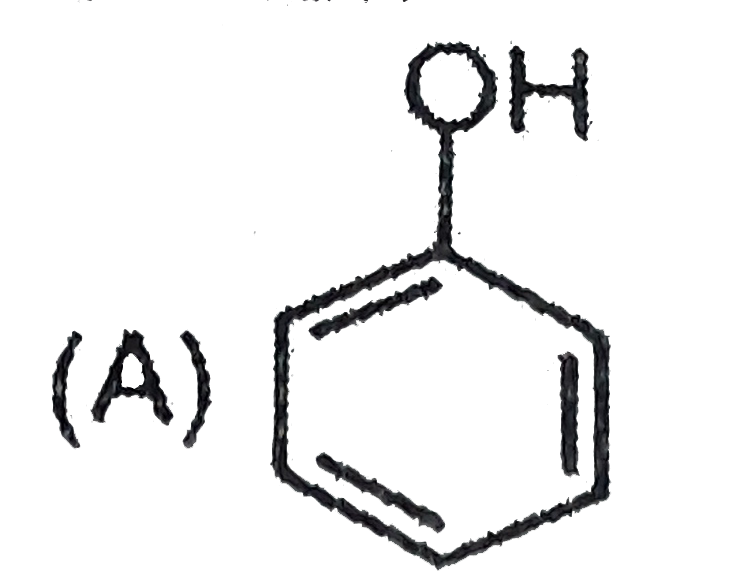
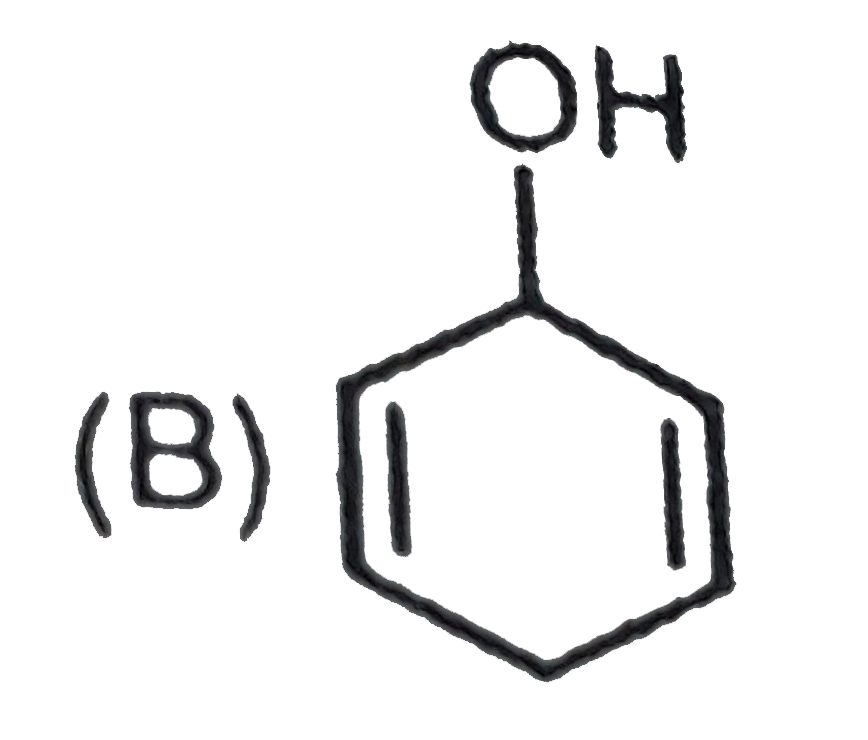
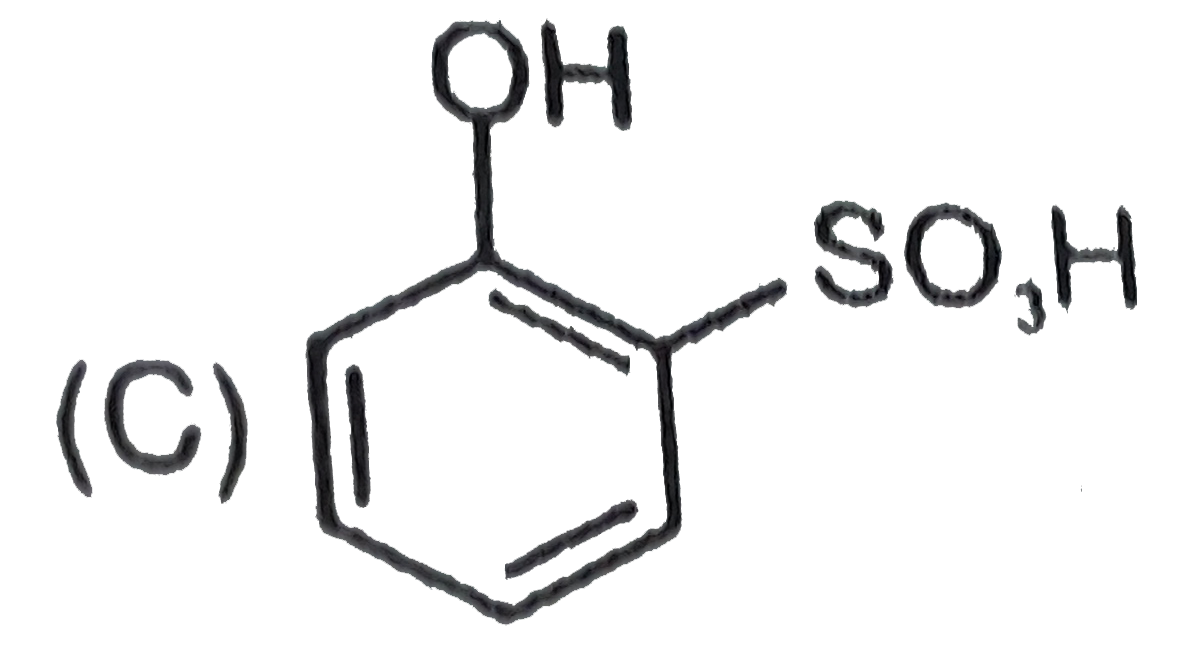
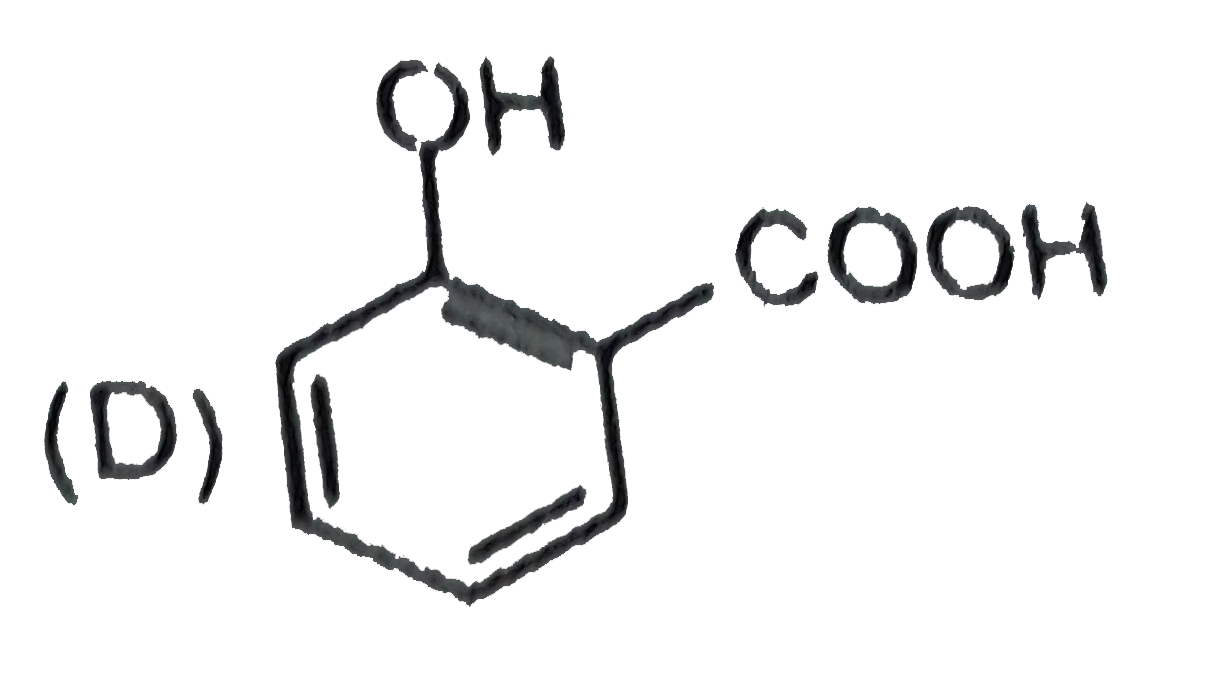
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
AROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise PART IV- COMPREHENSION|13 VideosAROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise Exercise -3 Part-I|36 VideosAROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise PART-II SINGLE OR DOUBLE INTEGER TYPE|7 VideosATOMIC STRUCTURE
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Fundamental Concept )|16 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-AROMATIC COMPOUNDS -PART-III ONE OR MORE THAN ONE OPTION CORRECT TYPE
- Which of the following can decolourise bromine water solution?
Text Solution
|
- Identify the intermediates of the following reaction.
Text Solution
|
- Select the correct options:
Text Solution
|
- Correct options for the given below reaction: Ph-OH overset((i) CHCl...
Text Solution
|
- Which of the following reaction is/are correct?
Text Solution
|
- Which of the following reaction is/are correct:
Text Solution
|
- The product of following sequences of reactions are
Text Solution
|
- R and S are
Text Solution
|
- Which of the following statements (s) for the above sequence of reacti...
Text Solution
|
- Coupling reaction takes place when benzene diazonium chloride is treat...
Text Solution
|