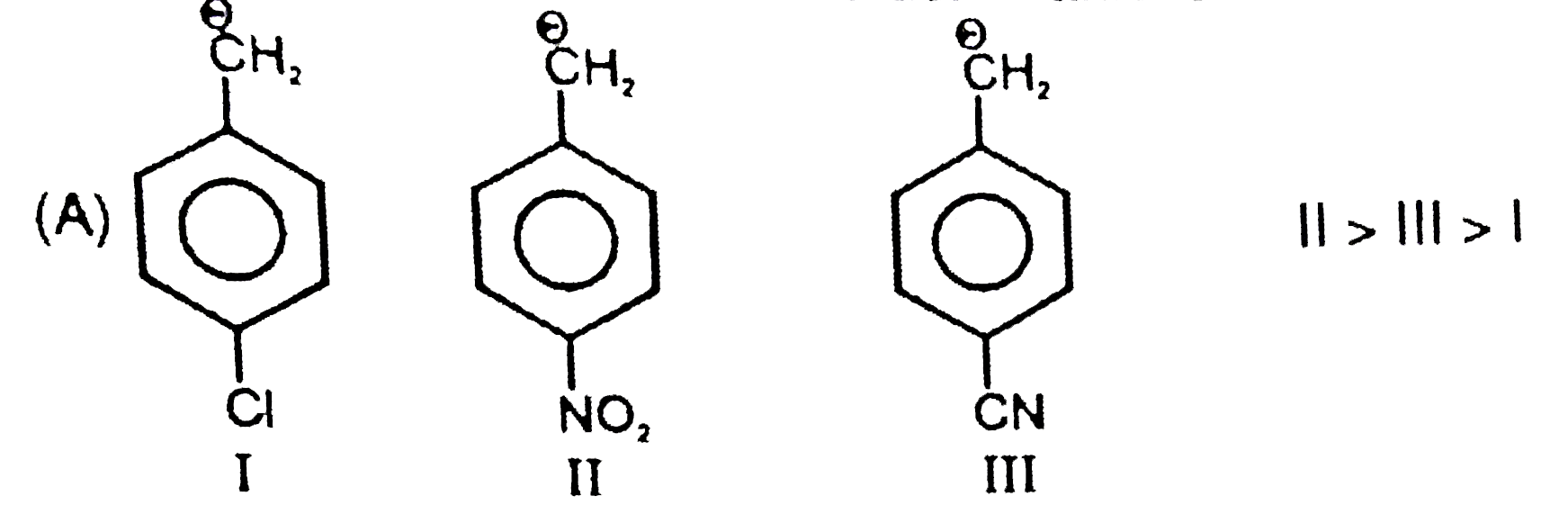
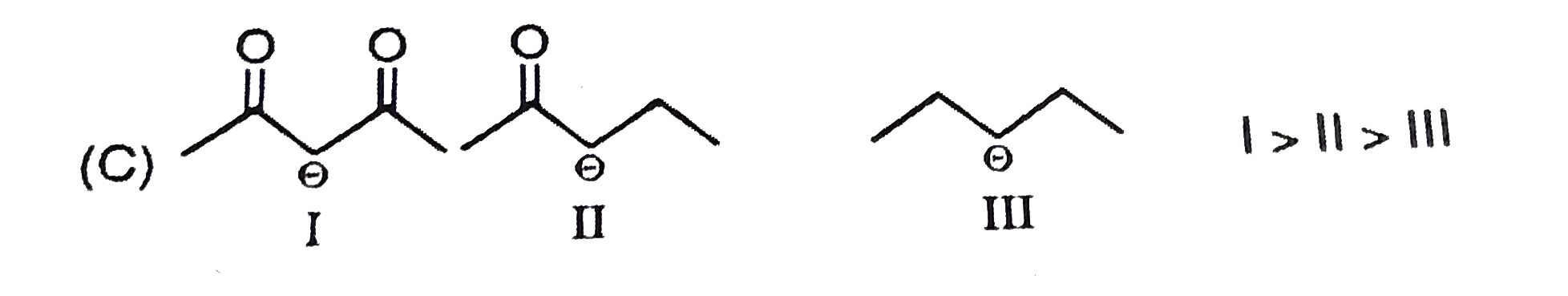
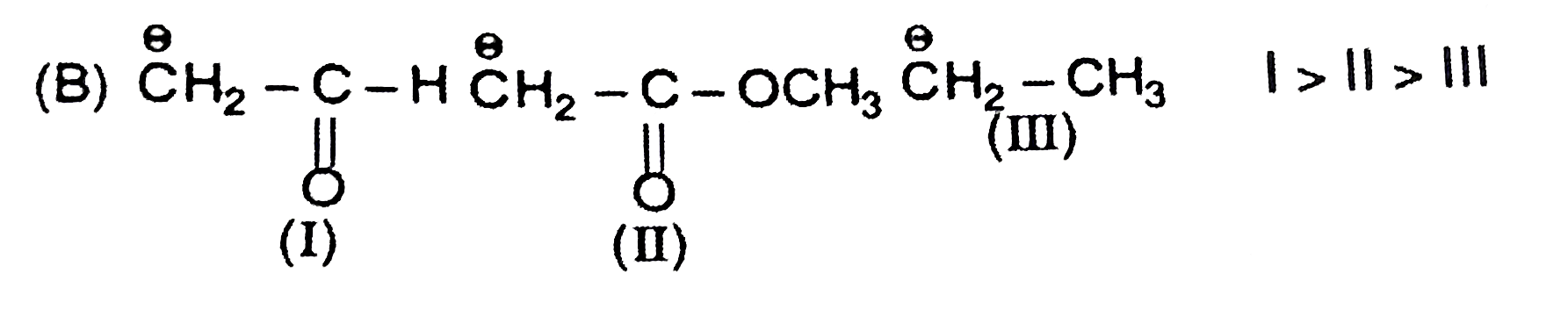A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY II
RESONANCE ENGLISH|Exercise Part-IV : Comprehension|12 VideosGENERAL ORGANIC CHEMISTRY II
RESONANCE ENGLISH|Exercise Exercise-3 Part:I JEE(Advanced)/IIT-JEE Problems (Previous Years)|17 VideosGENERAL ORGANIC CHEMISTRY II
RESONANCE ENGLISH|Exercise Part-II: Single And Double Value Integer Type|9 VideosGASEOUS STATE
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Hydrocarbon)|18 VideosGENERAL ORGANIC CHEMISTRY-I
RESONANCE ENGLISH|Exercise PART-III : PRACTICE TEST-19|1 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-GENERAL ORGANIC CHEMISTRY II-Part-III: One or More Than One Options Correct Type
- Which of the following stability order of anions is/are correct :
Text Solution
|
- Which of the following is/are correct for basic strength:
Text Solution
|
- Among the following which statement(s) is/are correct:
Text Solution
|
- Consider the following compounds O(2)N-CH(2)-underset((I))overset(O...
Text Solution
|
- Carbolic acid is less acidic than:
Text Solution
|
- Observe the compound and choose correct statement:
Text Solution
|
- Which of the following reactions favour backward direction ?
Text Solution
|
- The correct statement(s) concerning the structure P,Q,R & S is/are
Text Solution
|
- Among the given pairs, in which pair second compound has less enol con...
Text Solution
|