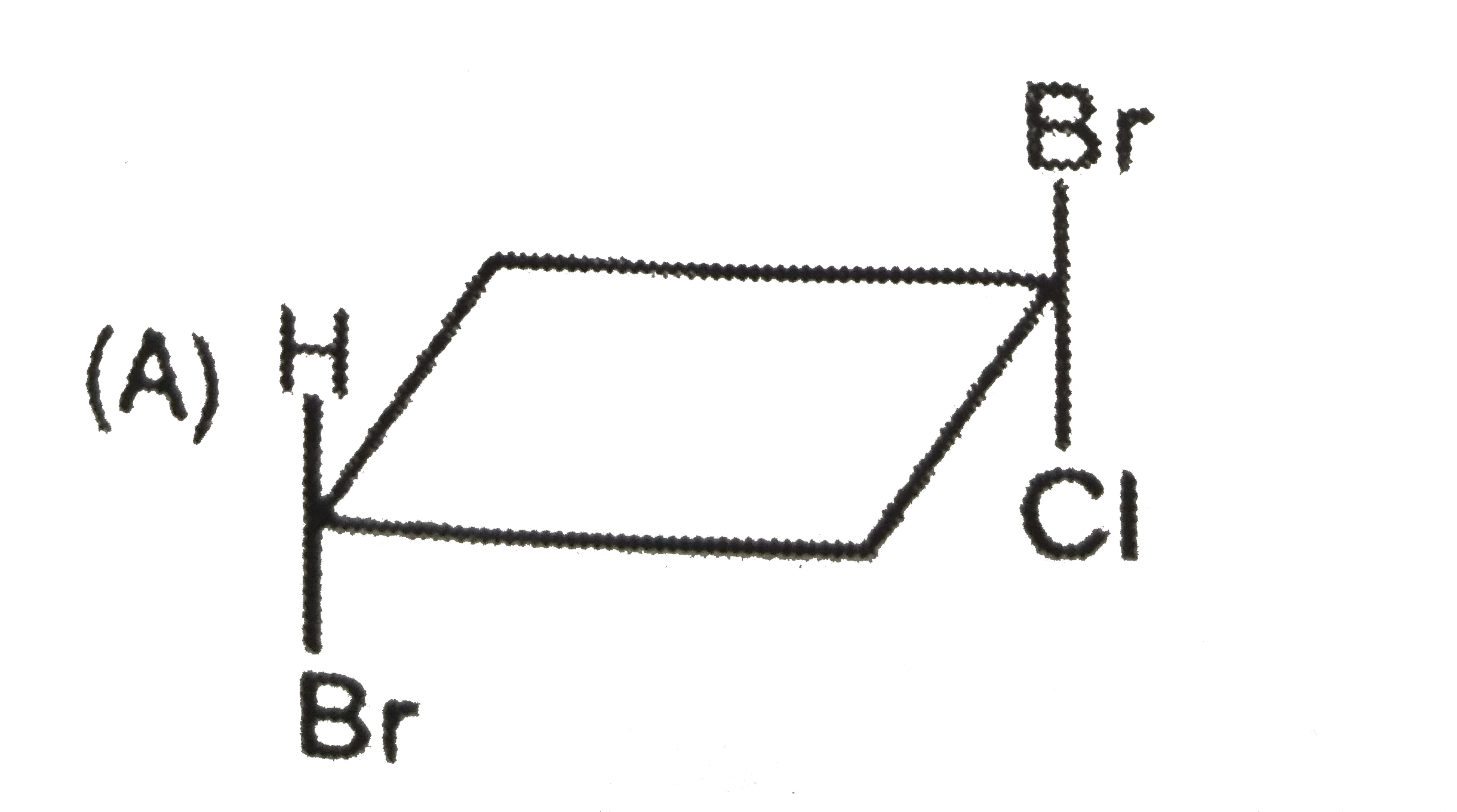
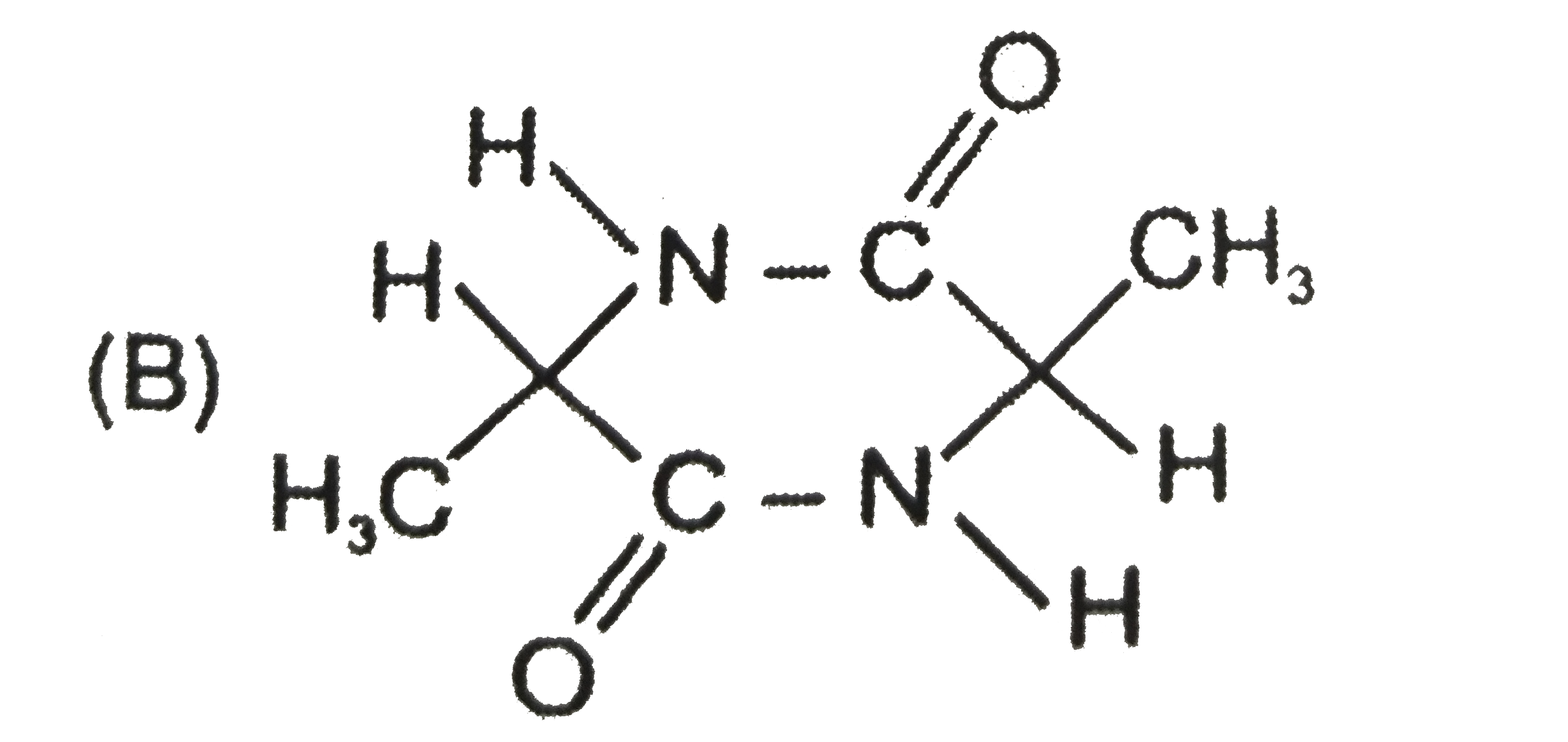
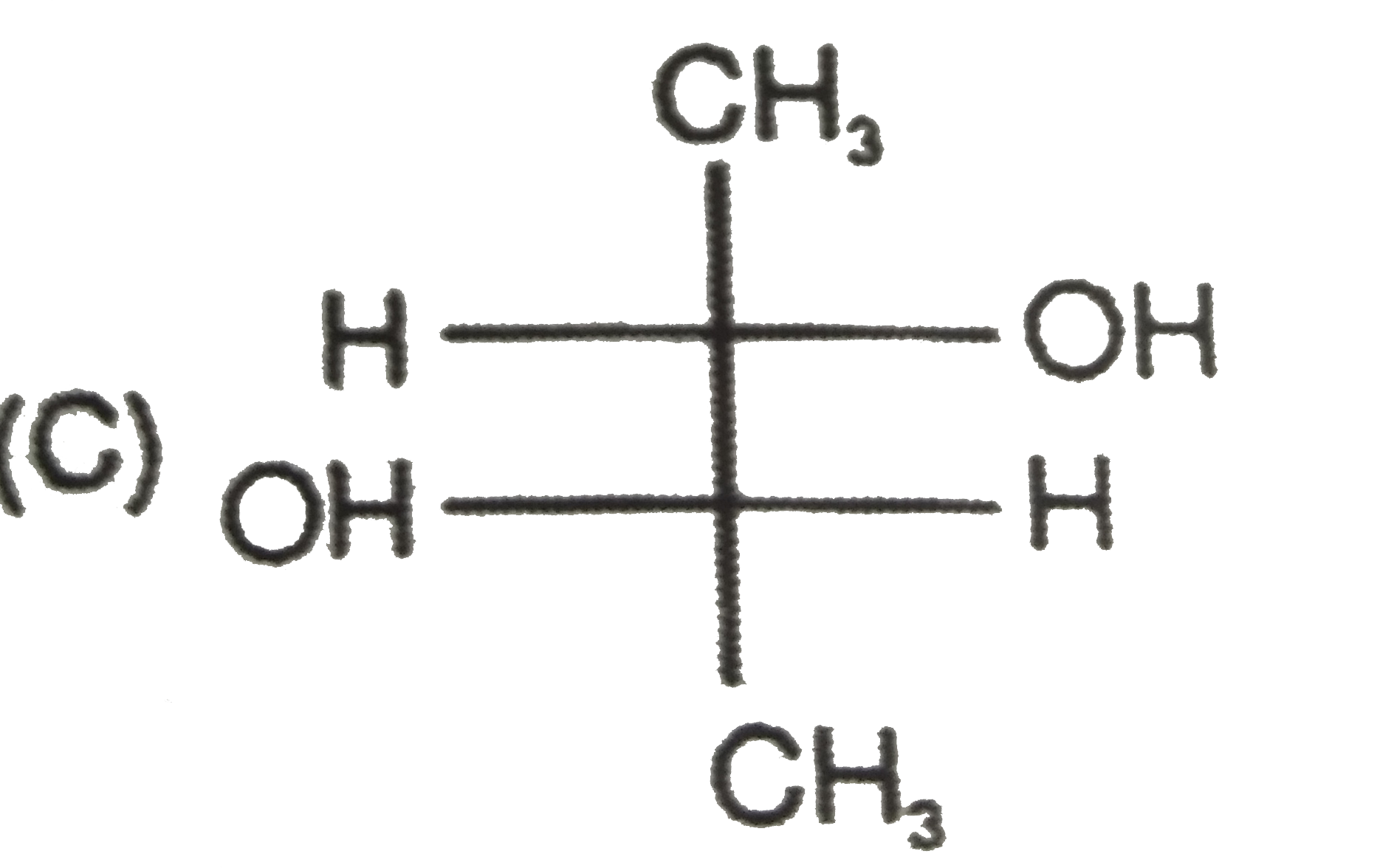
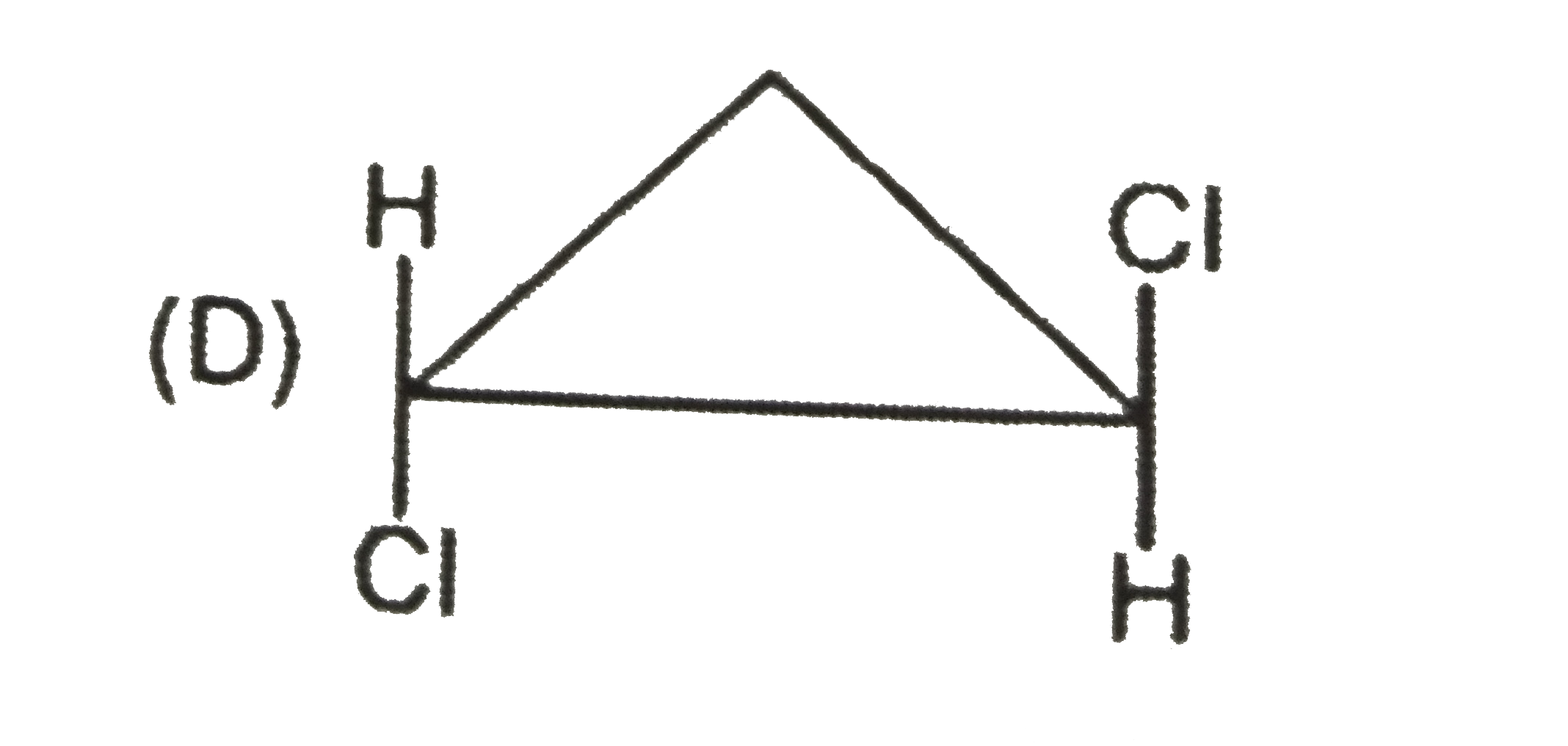
To determine which of the given compounds possesses a center of symmetry, we need to understand the concept of a center of symmetry (also known as an inversion center). A molecule has a center of symmetry if for every atom at position (x, y, z), there is an equivalent atom at position (-x, -y, -z). This means that the molecule can be reflected through a central point, and the parts of the molecule will match up perfectly.
### Step-by-Step Solution:
1. **Understand the Definition**:
- A center of symmetry exists in a molecule if for every atom in the molecule, there is an equivalent atom located at an equal distance from the center but in the opposite direction.
2. **Analyze Each Compound**:
- We need to examine the structures of the compounds A, B, C, and D to see if they meet the criteria for having a center of symmetry.
3. **Examine Compound B**:
- The structure of compound B is given as Nc=O (carbonyl), H, C≡N (nitrile), CH3, H, and CO.
- When we analyze this structure, we can see that there are equivalent groups present. For example, the presence of two hydrogen atoms and two CH3 groups indicates symmetry.
4. **Reflection Through the Center**:
- If we take the center of compound B as the point of reflection, we can see that each group can be reflected back through this point at equal distances but in opposite directions.
5. **Conclusion**:
- Since compound B has equivalent groups and can be reflected through a central point, it possesses a center of symmetry.
6. **Final Answer**:
- Therefore, the compound that possesses a center of symmetry is **Option B**.