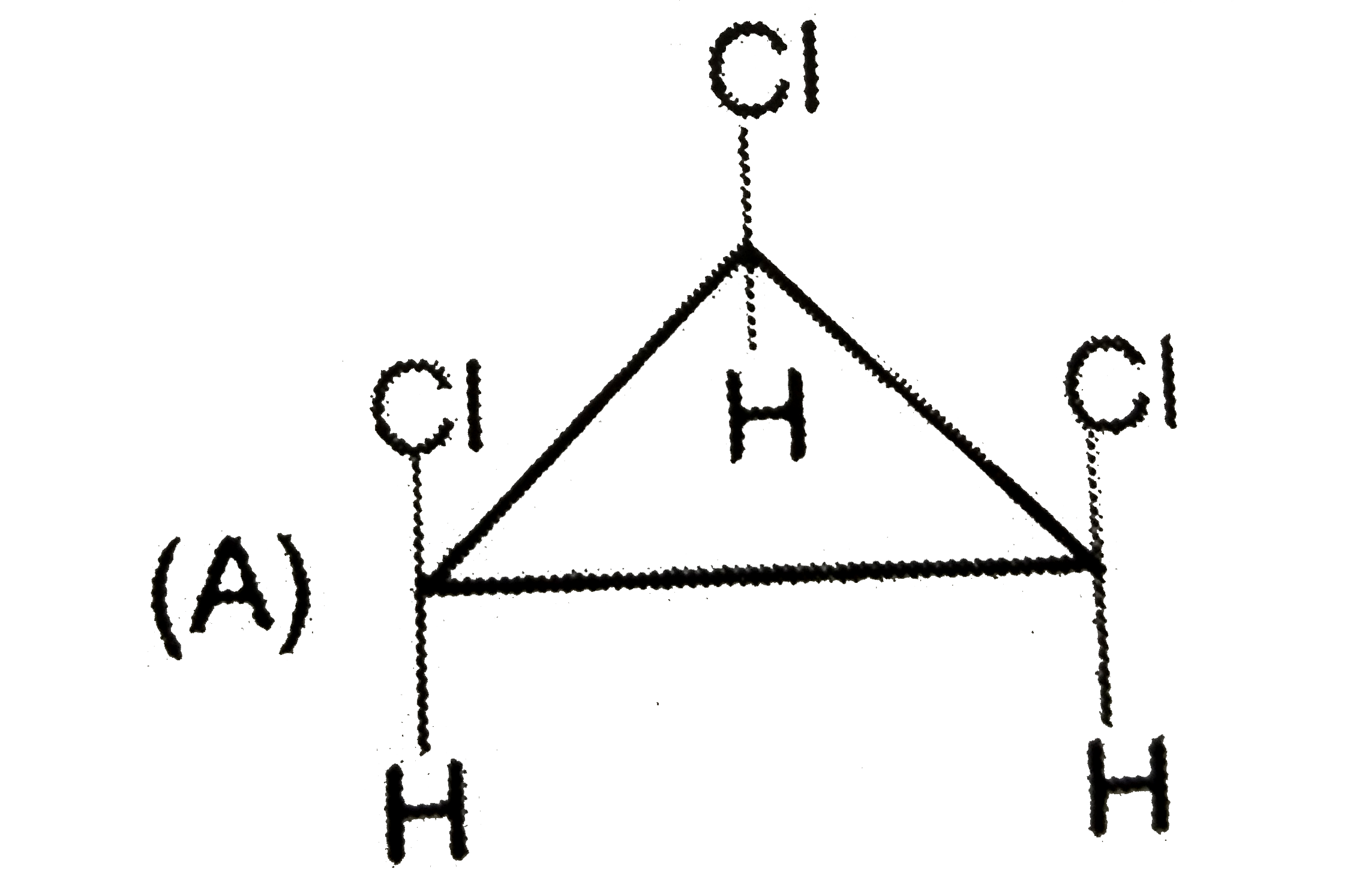
A
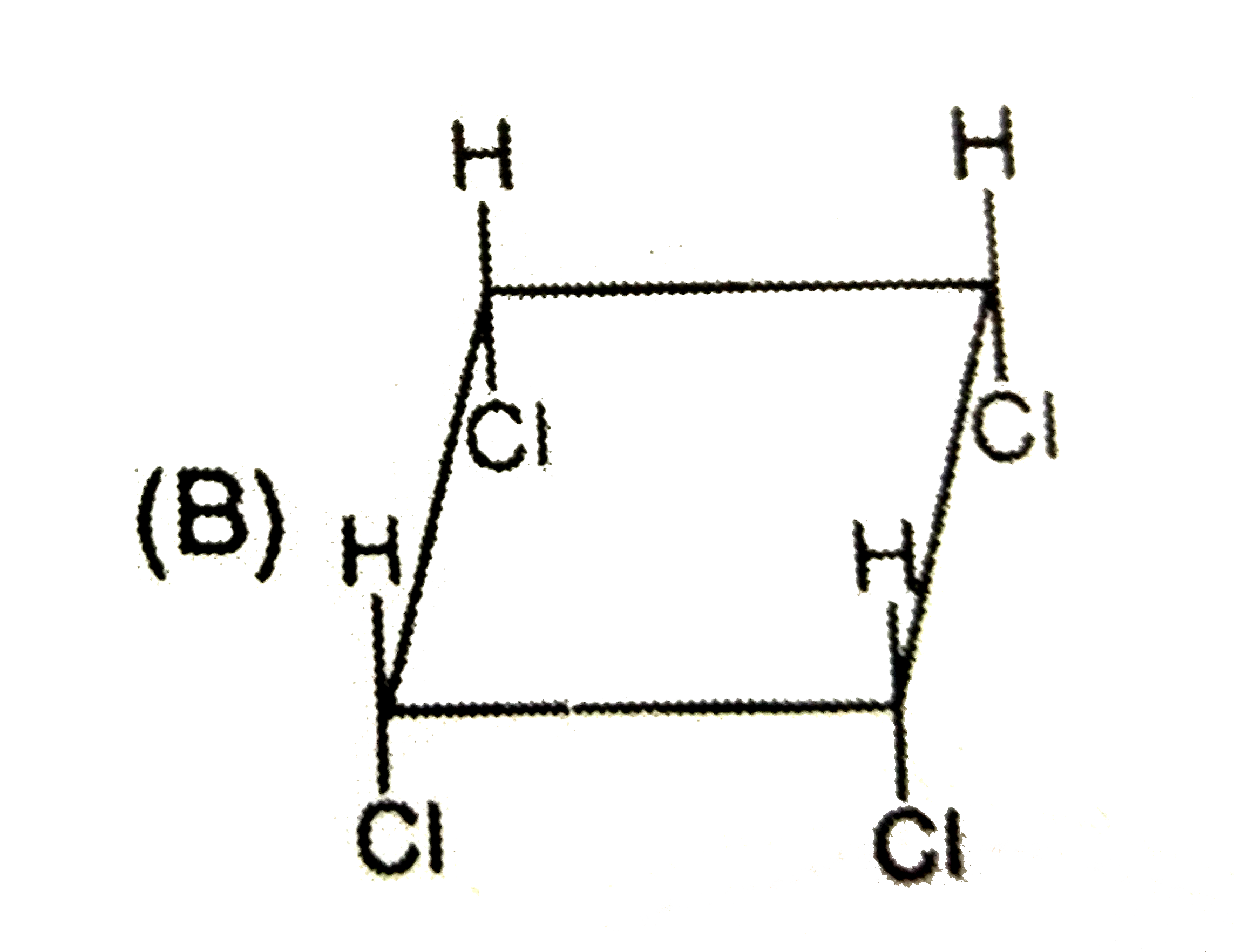
B
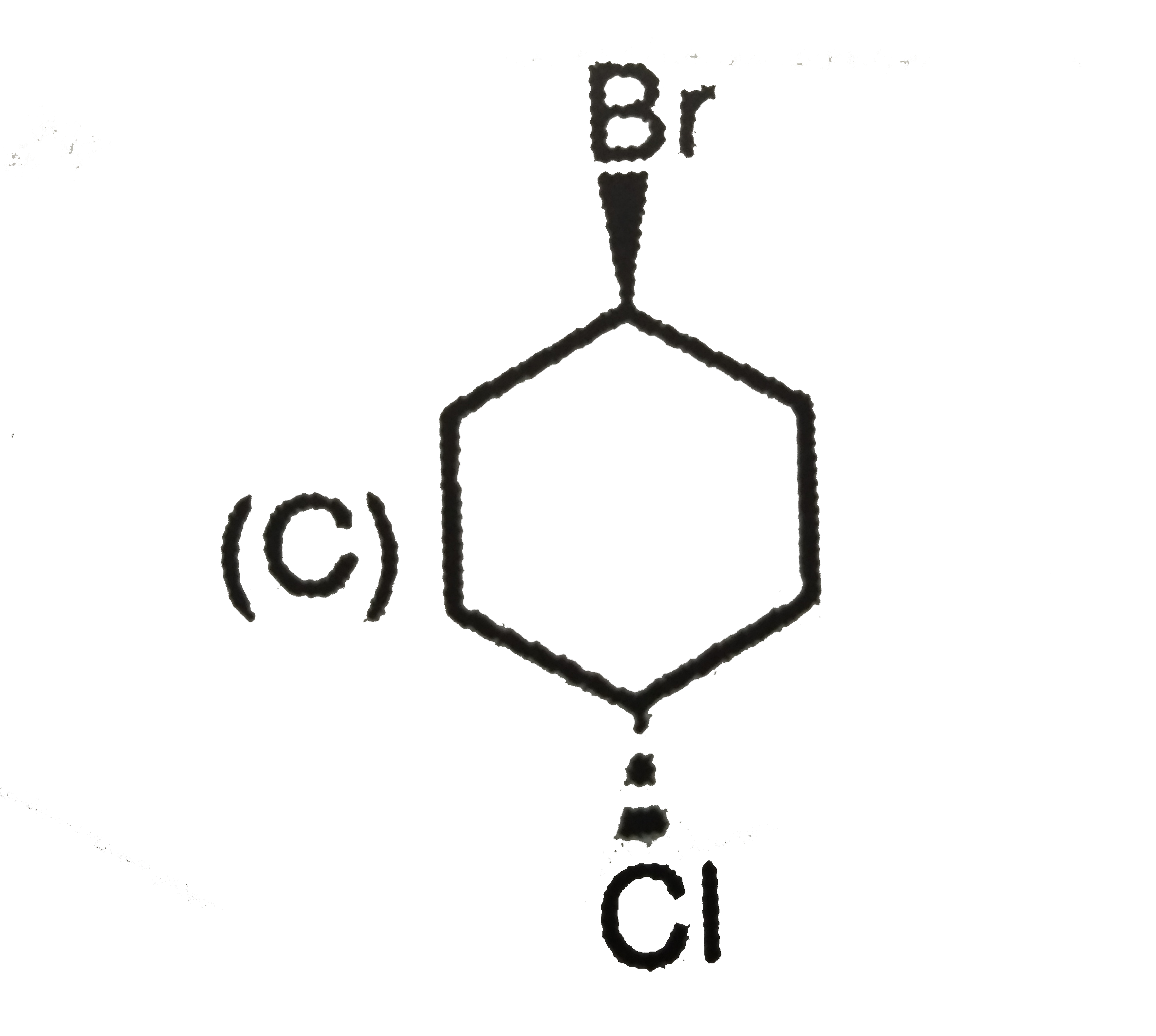
C
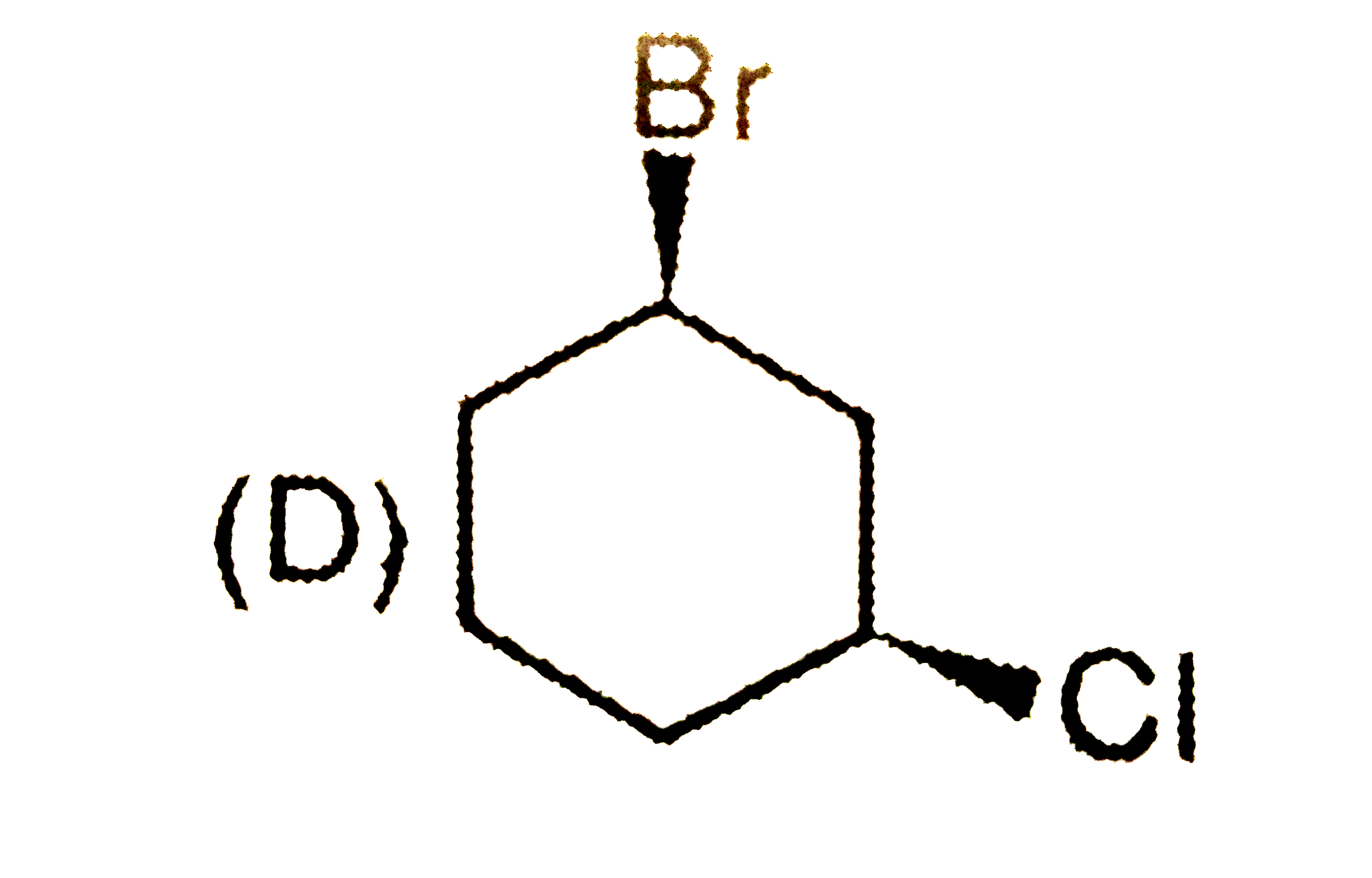
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
STEREOISOMERISM
RESONANCE ENGLISH|Exercise EXERCISE (PART II : SINGLE AND DOUBLE VALUE INTEGER TYPE)|15 VideosSTEREOISOMERISM
RESONANCE ENGLISH|Exercise EXERCISE (PART III : ONE OR MORE THAN ONE OPTIONS CORRECT TYPE)|11 VideosSTEREOISOMERISM
RESONANCE ENGLISH|Exercise EXERCISE (PART III MATCH THE COLUMN)|1 VideosSOLUTIONS
RESONANCE ENGLISH|Exercise Advabced Level Problems (PART-2)|35 VideosSTRUCTURAL IDENTIFICATION
RESONANCE ENGLISH|Exercise Advanced level Problems (Part-III)|12 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-STEREOISOMERISM-EXERCISE (PART I ONLY ONE OPTION CORRECT TYPE)
- Which of the following molecule is chiral.
Text Solution
|
- Which one of the following compounds will show enantiomerism ?
Text Solution
|
- Which of the following statements regarding the projections shown belo...
Text Solution
|
- The structures represent
Text Solution
|
- The given compound (X) has:
Text Solution
|
- The compound X and Y in below reaction can be Ph-NH*NH(2)+ (X) + (Y) o...
Text Solution
|
- How many optical isomers are possible for following compound ?
Text Solution
|
- How many stereoisomers are possible for
Text Solution
|
- How may spatial orientations are possible in the following compound ?
Text Solution
|
- Number of conformational isomers of ethane.
Text Solution
|