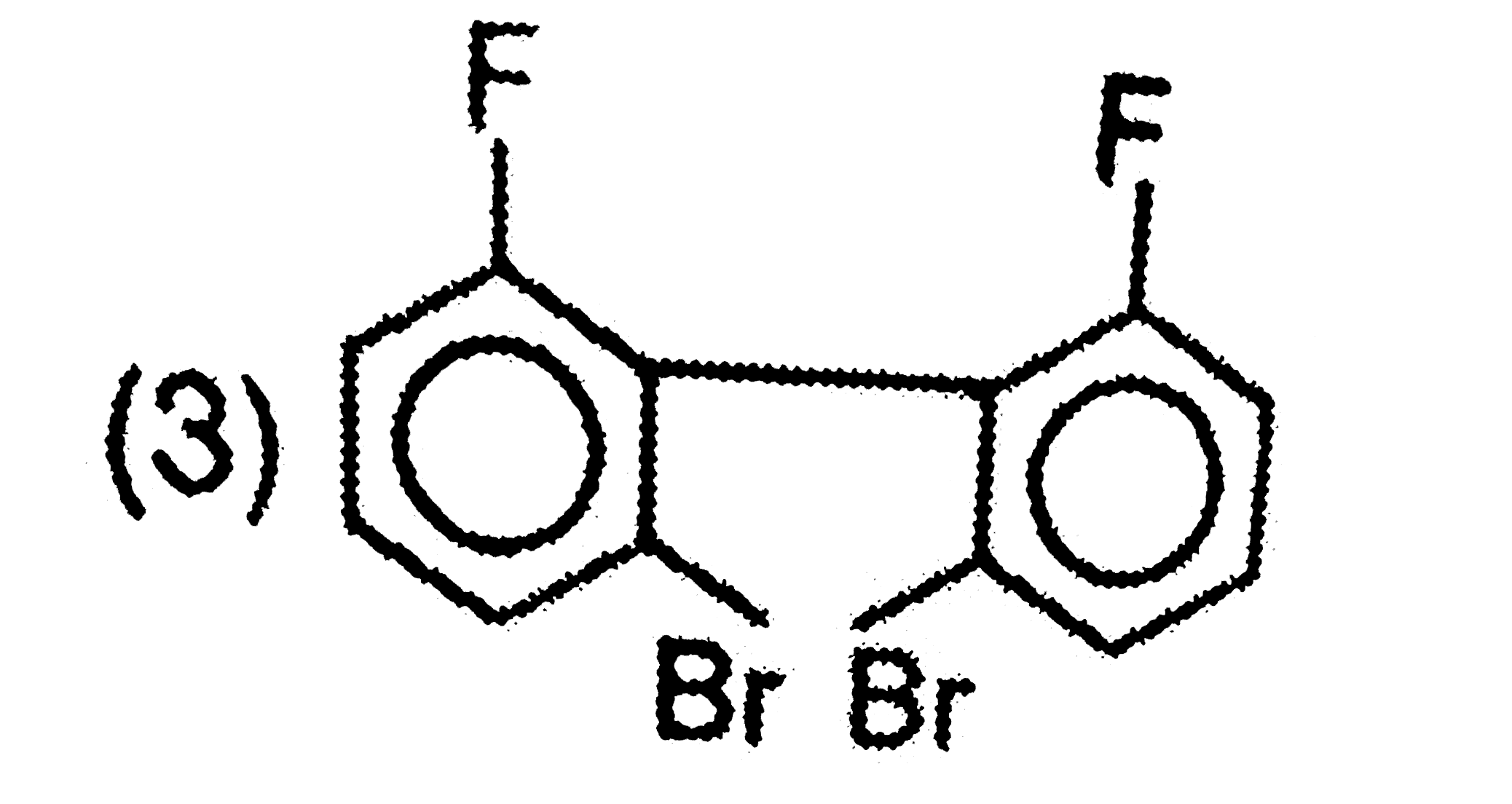
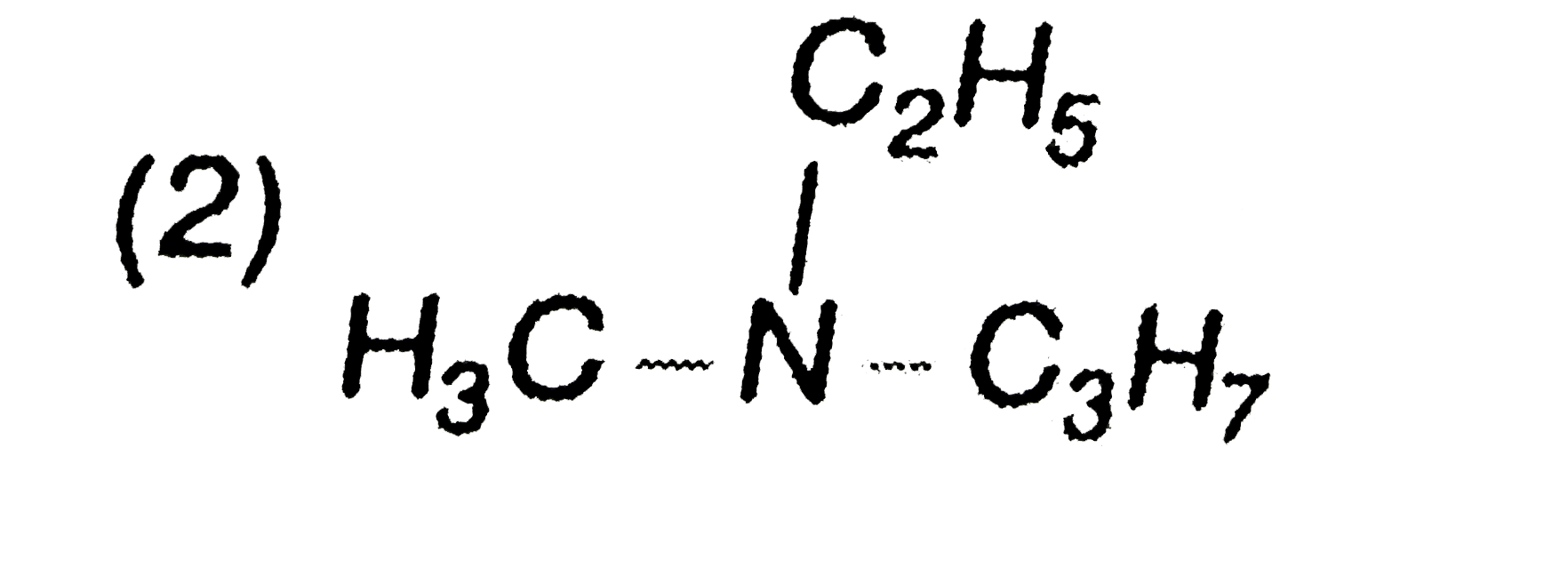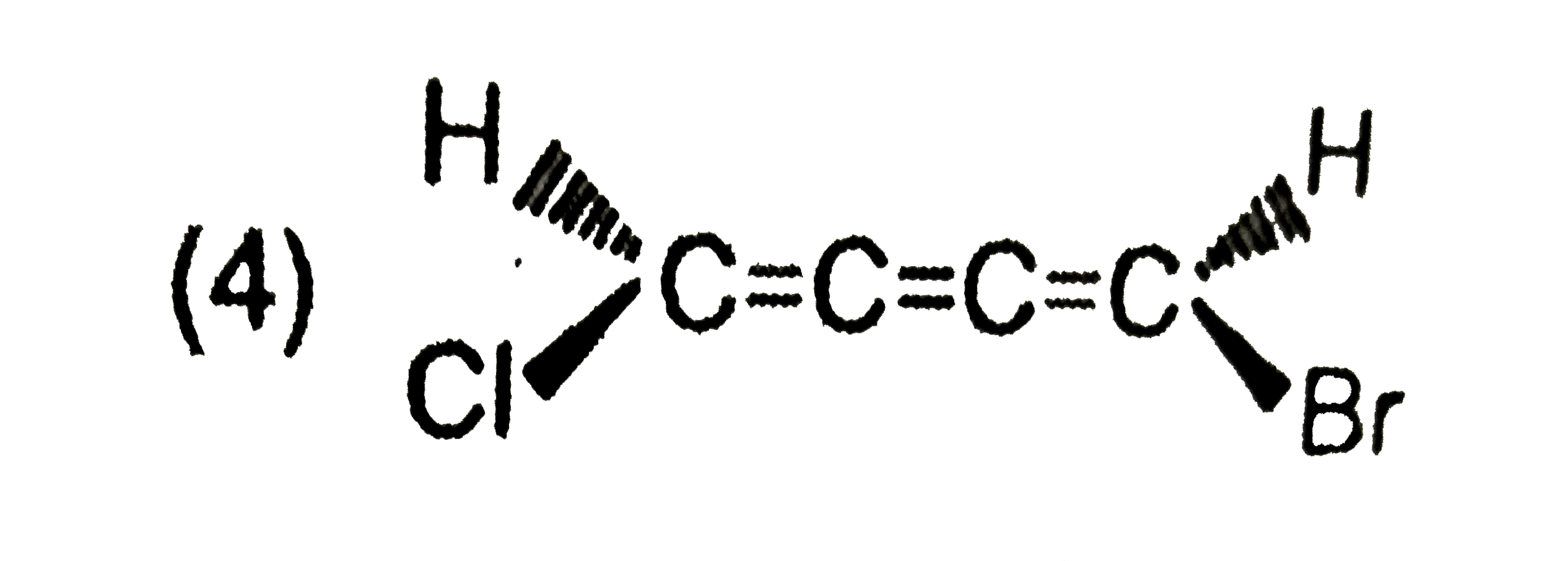A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
STEREOISOMERISM
RESONANCE ENGLISH|Exercise EXERCISE (PART II : NATIONAL STANDARD EXAMINATION IN CHEMISTRY (NSEC) STAGE-1)|55 VideosSTEREOISOMERISM
RESONANCE ENGLISH|Exercise EXERCISE (PART III : PRACTICE TEST-2 (IIT-JEE (ADVANCED PATTERN))|23 VideosSTEREOISOMERISM
RESONANCE ENGLISH|Exercise EXERCISE (PART -II ONLINE JEE MAIN)|3 VideosSOLUTIONS
RESONANCE ENGLISH|Exercise Advabced Level Problems (PART-2)|35 VideosSTRUCTURAL IDENTIFICATION
RESONANCE ENGLISH|Exercise Advanced level Problems (Part-III)|12 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-STEREOISOMERISM-EXERCISE (ADDITIONAL PROBLEMS FOR SELF PRACTICE (APSP)) PART 1
- Which type of isomerism is shown by 2, 3-Dichlorobutane ?
Text Solution
|
- Increasing order of stability among the three main conformation ( i.e....
Text Solution
|
- The unusually stable three membered unsaturated compound, Feist acid w...
Text Solution
|
- Which of the following depict the same ?
Text Solution
|
- The total number of chiral centres present in the artificial sweetener...
Text Solution
|
- Which pair is identical ?
Text Solution
|
- Identify the relation between molecules given in Newman and Fischer pr...
Text Solution
|
- The correct IUPAC name of D-Glucose is :
Text Solution
|
- Which of the following species will be optically active ?
Text Solution
|
- Which of the following compounds exhibits stereoisomerism?
Text Solution
|
- The isomeric alcohol which has a chiral carbon atom is :1
Text Solution
|
- Which of the following are intensive properties?
Text Solution
|
- The R/S designation for the following stereoisomers of 1,3-dichloro-2-...
Text Solution
|
- Which of the following will not show geometrical isomerism?
Text Solution
|
- The racemic mixture in liquid/gaseous state will have
Text Solution
|
- True statement(s) regarding the given molecule is/are
Text Solution
|
- The most stable conformation of cyclohexane is
Text Solution
|
- The two compounds (I) and (II) are related as :
Text Solution
|
- How many stereoisomers of a drug for healing the wounds are possible &...
Text Solution
|
- Which of the following compunds is capable of showing geometrical, op...
Text Solution
|