A
B
C
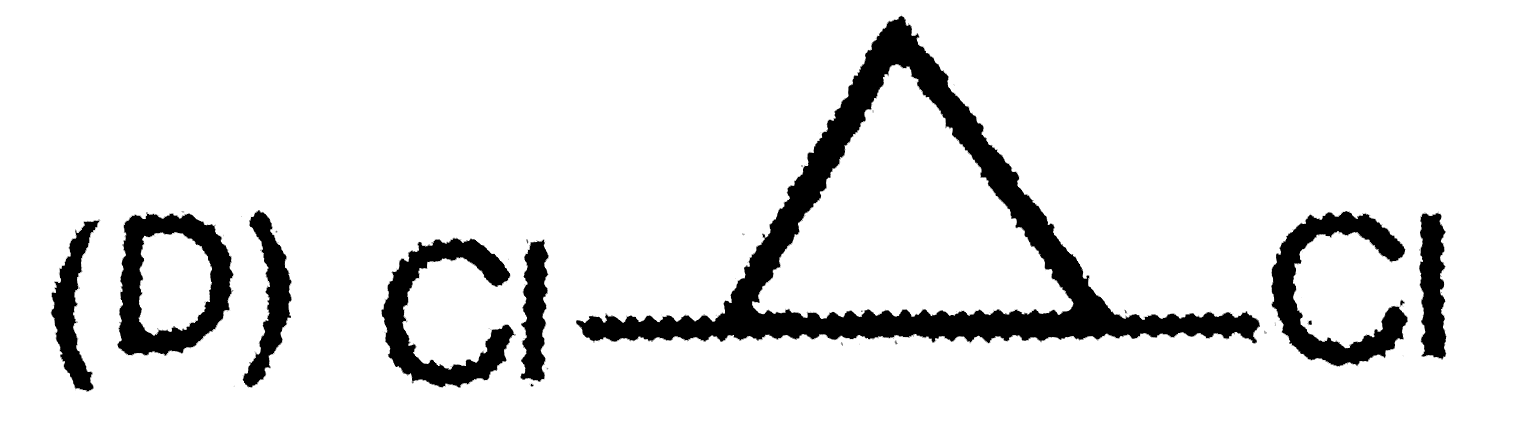
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
STEREOISOMERISM
RESONANCE ENGLISH|Exercise EXERCISE (PART III : PRACTICE TEST-2 (IIT-JEE (ADVANCED PATTERN))|23 VideosSTEREOISOMERISM
RESONANCE ENGLISH|Exercise EXERCISE (ADDITIONAL PROBLEMS FOR SELF PRACTICE (APSP)) PART 1|30 VideosSOLUTIONS
RESONANCE ENGLISH|Exercise Advabced Level Problems (PART-2)|35 VideosSTRUCTURAL IDENTIFICATION
RESONANCE ENGLISH|Exercise Advanced level Problems (Part-III)|12 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-STEREOISOMERISM-EXERCISE (PART II : NATIONAL STANDARD EXAMINATION IN CHEMISTRY (NSEC) STAGE-1)
- What are enantiomers
Text Solution
|
- The number of all types of isomers of chlorobutane is :
Text Solution
|
- (i) CH(2)=CH-CH(2)-CH=CH(2) (ii) CH(2)=CH-CH=CH-CH(3) (iii) CH(3)-...
Text Solution
|
- The configurations of the carbon atoms C(2) and C(3) in the following ...
Text Solution
|
- The compound that is chiral
Text Solution
|
- The number of stereoisomers of compound CH(3)-CH=CH-CH(Br)CH(3) is :
Text Solution
|
- The R/S designation for the following stereoisomers of 1,3-Dibromo-2-m...
Text Solution
|
- Among the isomers of Dimethylcyclohexanes, the chiral ones are
Text Solution
|
- Which one of the following compound has R configuration ?
Text Solution
|
- The numer of optically active stereoisomers of tartaric acid, (HOOC.CH...
Text Solution
|
- The above structures are related to each other as
Text Solution
|
- Which of the following molecules cannot show geometric isomerism ?
Text Solution
|
- 2-methylpentane is :
Text Solution
|
- The two projection formulae that represent a pair of enantiomers are.
Text Solution
|
- The Fischer projection formula that represents the following compounds...
Text Solution
|
- Levonorgestrel is a commonly used contraceptive. The number of chiral ...
Text Solution
|
- Number of stereoisomers possible for the following compound is
Text Solution
|
- The numer of quaternary and chiral carbon atoms present in elatol, iso...
Text Solution
|
- The Newman projection shown is the same as
Text Solution
|
- The molecule in which all atoms are not coplanar is
Text Solution
|