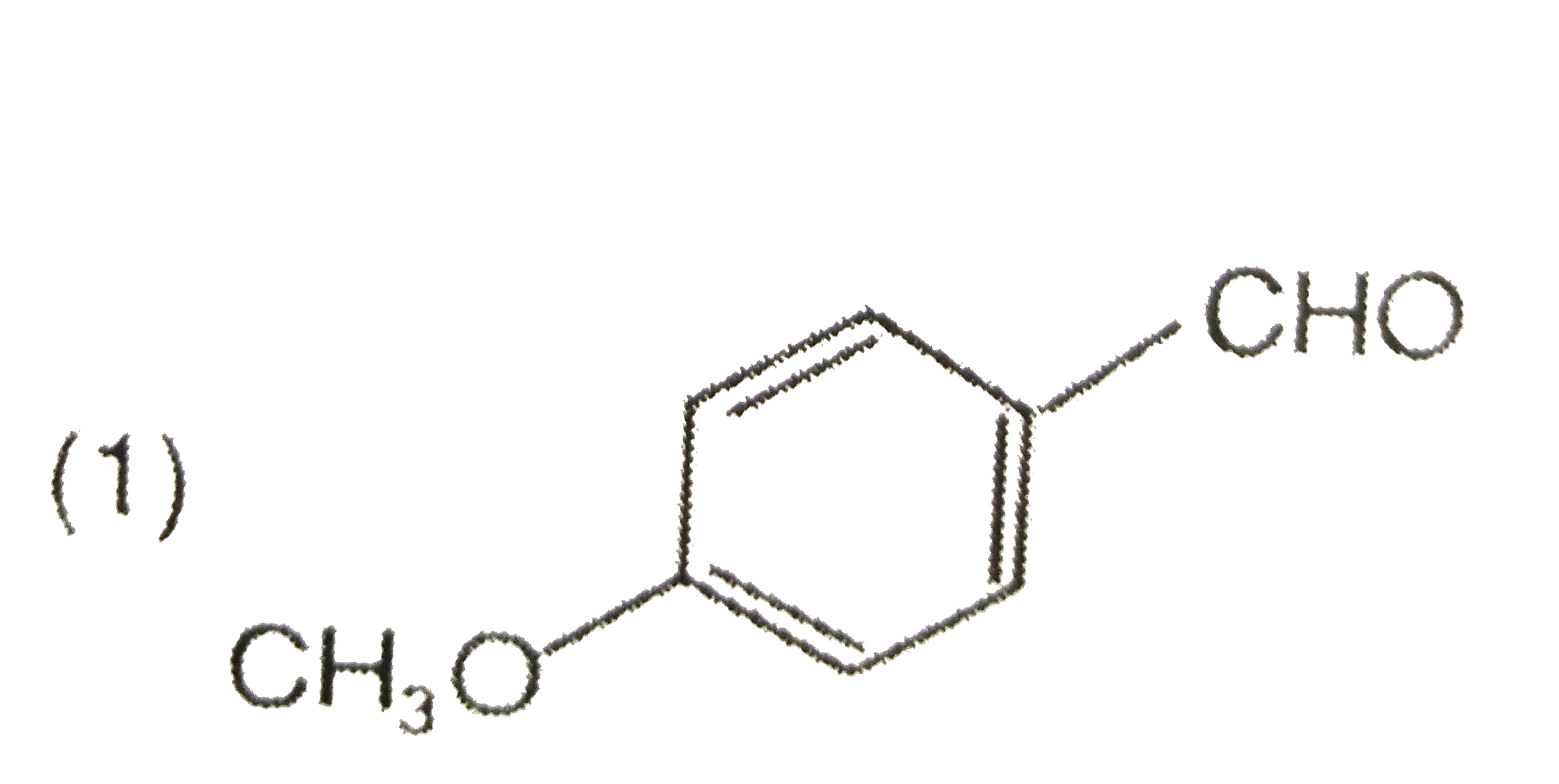
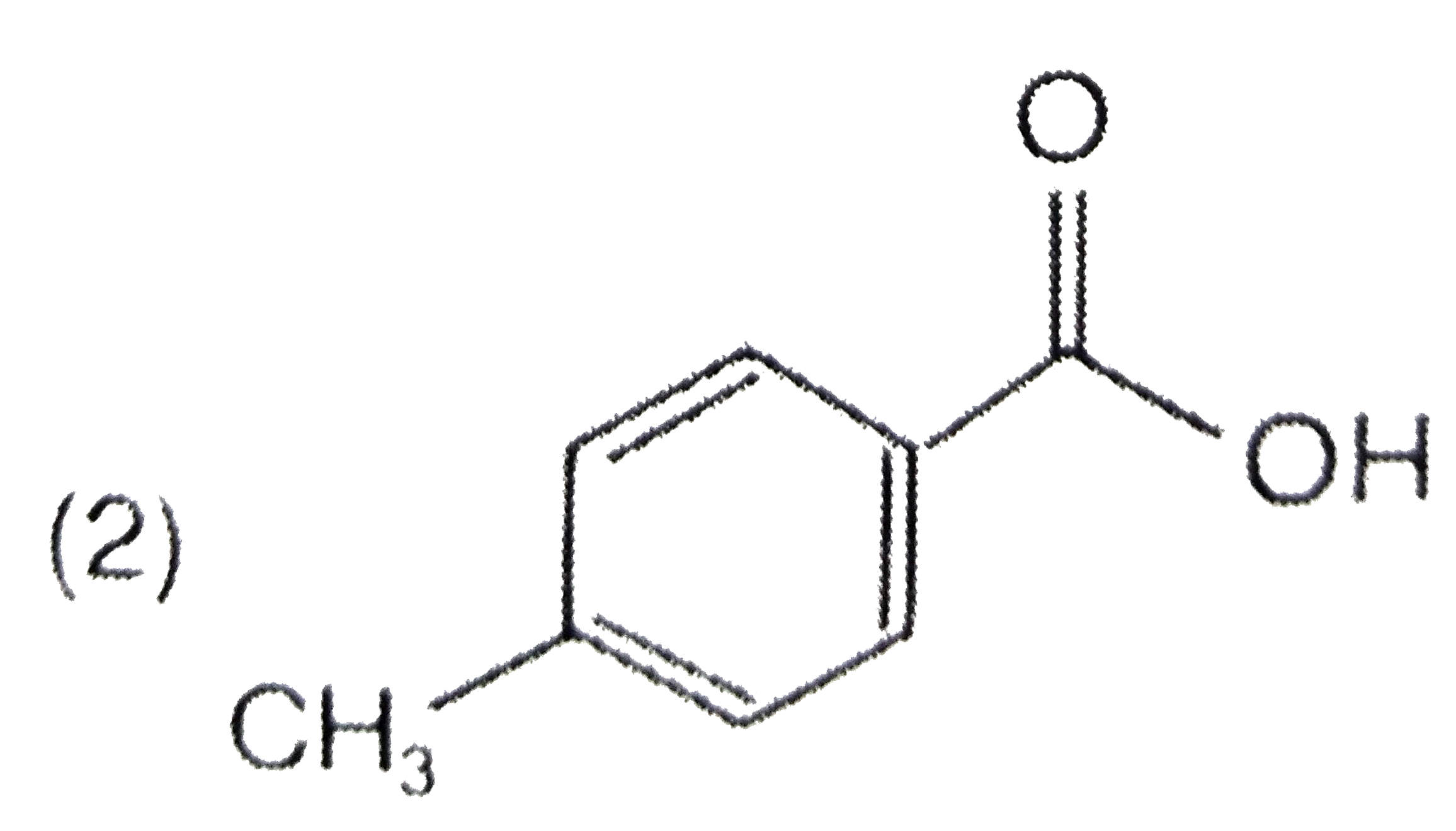
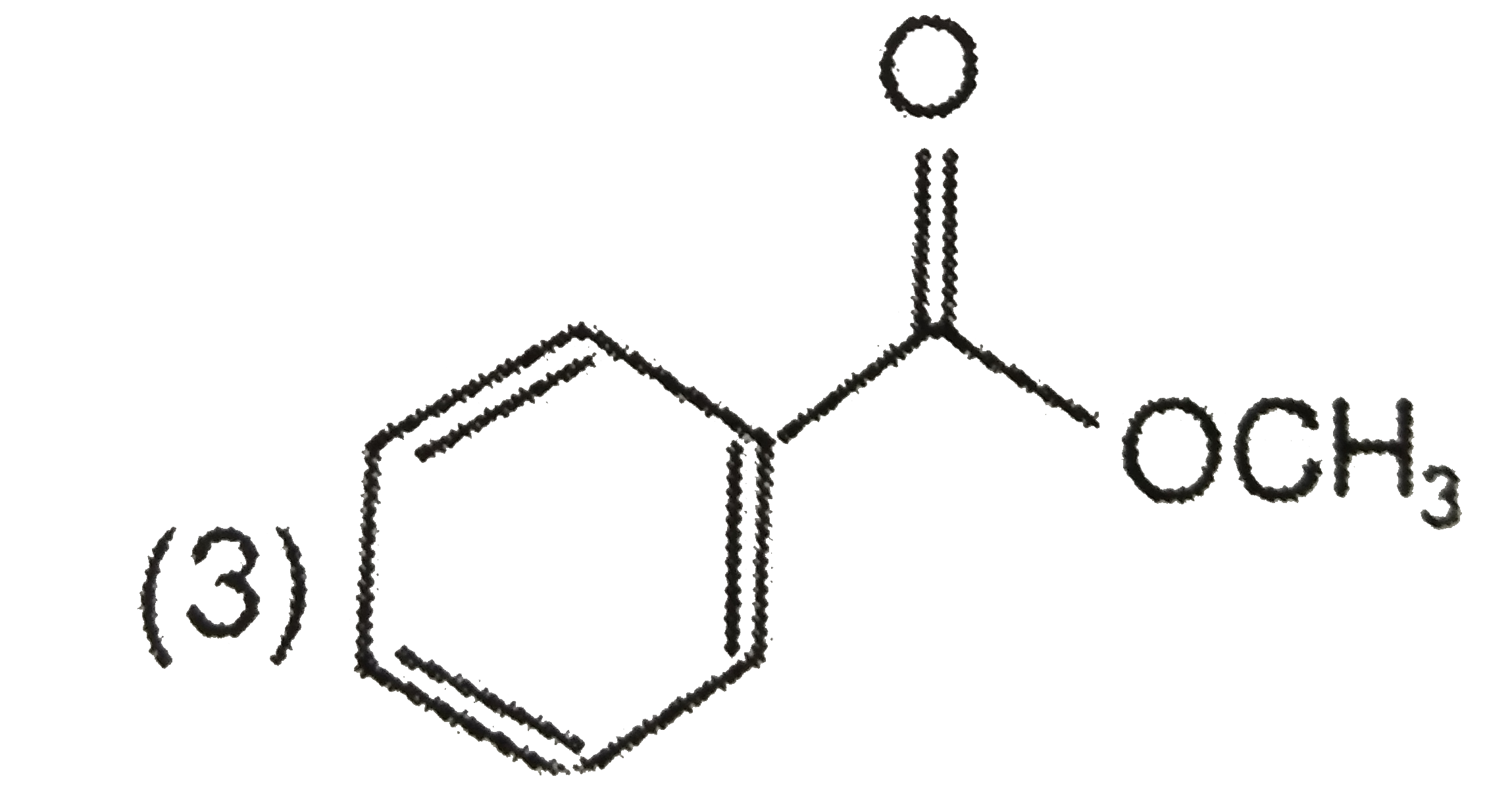
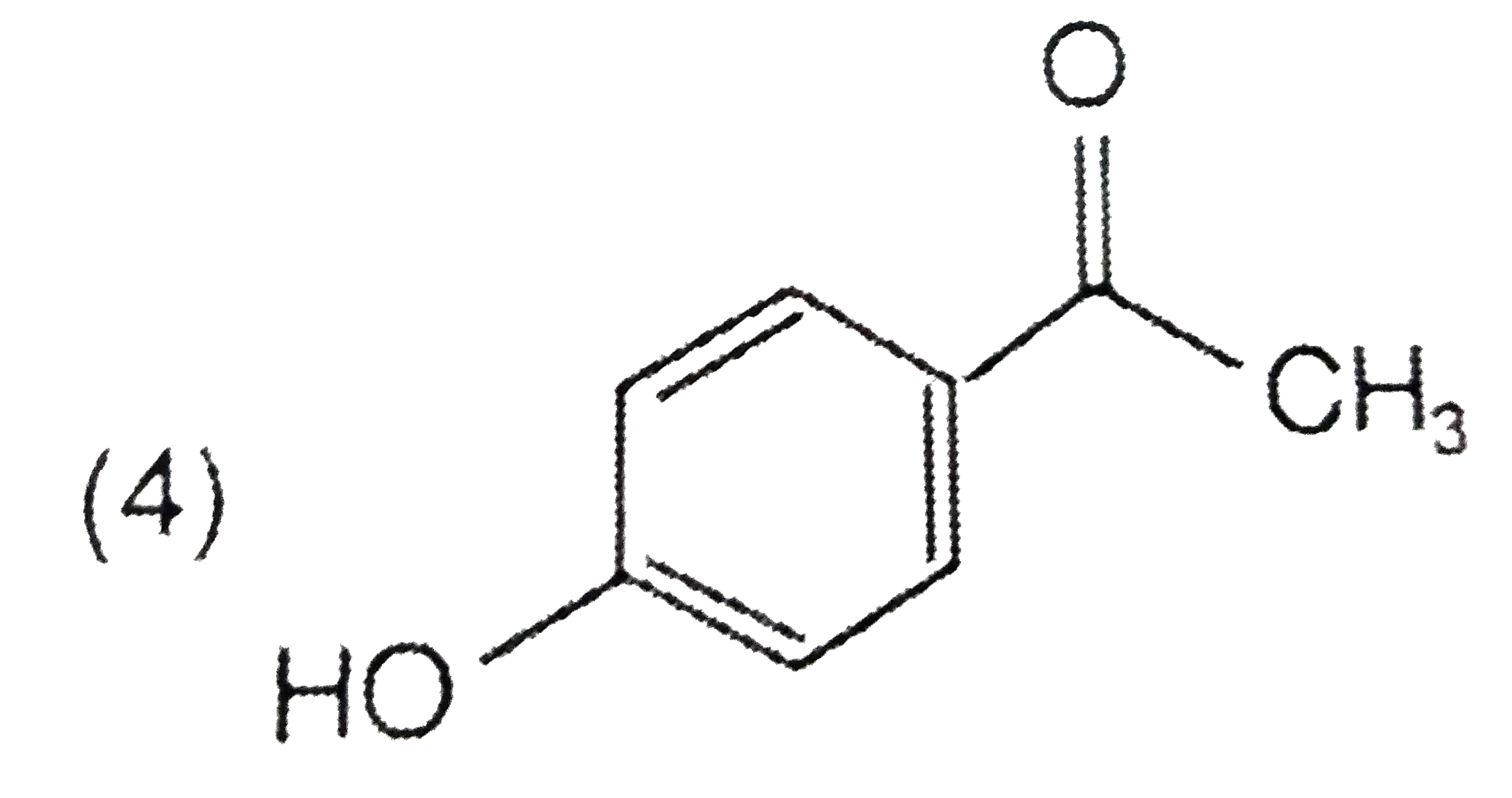
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID
RESONANCE ENGLISH|Exercise Part-III|8 VideosCARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID
RESONANCE ENGLISH|Exercise Exercise-2 Part-I|1 VideosCARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID
RESONANCE ENGLISH|Exercise Exercise-1 Part-II|1 VideosBIOMOLECULES & POLYMER
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Biomolecules & Polymer)|34 VideosCHEMICAL KINETICS
RESONANCE ENGLISH|Exercise PHYSICAL CHEMITRY (CHEMICAL KNIETICS & RADIOACTIVITY)|49 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID -Part-II
- CH(3)CH(2)COOHoverset(Cl(2))underset("red P")rarrAoverset("alc. KOH")r...
Text Solution
|
- p-Cresol reacts with chloroform in alkaline medium to give the compoun...
Text Solution
|
- An organic compound having molecular mass 60 is found to contain C = 2...
Text Solution
|
- A liquid was mixed with ethanol and a drop of concentrated H(2)SO(4) ...
Text Solution
|
- A compound with molecular mass 180 is acylated with CH(3)COCl to get a...
Text Solution
|
- Compound (A), C(8)H(9)Br, gives a white precipitate when warmed with a...
Text Solution
|
- Which of the following reactions will not result in the formation of c...
Text Solution
|
- Tischenko reaction.
Text Solution
|
- A compound A with molecular formula C(15)H(13)Cl gives a white precipi...
Text Solution
|
- In the reaction sequences 2CH(3)CHO overset(OH^(-))toA overset(Delta...
Text Solution
|
- The correct statement about the synthesis of erythritol (C(CH2OH)4) us...
Text Solution
|
- A compound of molecular formula C(8)H(8)O(2) reacts with acetophenon...
Text Solution
|
- The major products formed in the following reactions is
Text Solution
|
- Which is the most suitable reagent for the following transformation ? ...
Text Solution
|
- In the following reactions, prodcut A and B are : .
Text Solution
|
- An organic compound A,C(5)H(8)O reacts with H(2)O, NH(3) and CH(3)COOH...
Text Solution
|
- Among the following organic acids, the acid present in rancid butter i...
Text Solution
|
- In the presence of a small amount of phosphorous, aliphatic carboxylic...
Text Solution
|
- Which dicarboxylic acid in presence of a dehydrating agent is least re...
Text Solution
|
- In the following reaction, Aldehyde +"Alcohol" overset(HCI)toAcetal. ...
Text Solution
|