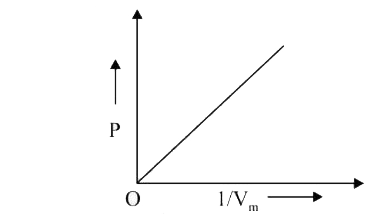
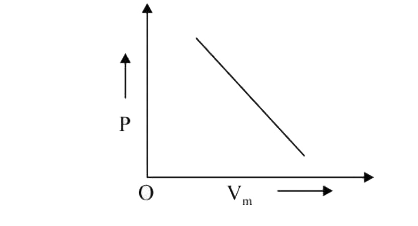
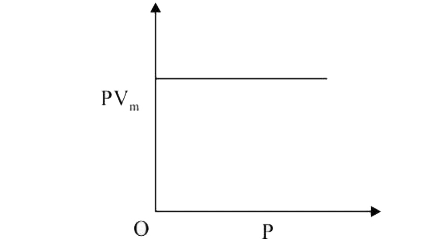
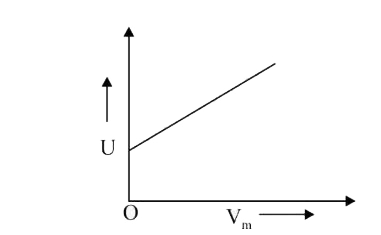
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-JEE MAIN REVISION TEST - 7|JEE - 2020-Chemistry (Section - 2) Numercial Value type
- The combination of plots which does not represent isothermal expansion...
Text Solution
|
- 2.44 g 'w' g of the benzoic acid dissolved in 30 g of benzene shows de...
Text Solution
|
- An open vessel at 27^@C is heated untill two fifth of the air (assumed...
Text Solution
|
- For a reaction, consider the plot of In K versus 1//T given in the fig...
Text Solution
|
- If K(sp) of Ag(2)CO(3) is 8 xx 10^(-12), the molar solubility of Ag(2)...
Text Solution
|
- The volume strength of g1 M H(2)O(2) is : (Molar mass of H(2)O(2) = 34...
Text Solution
|