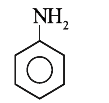
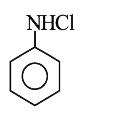
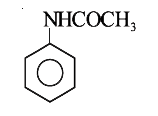
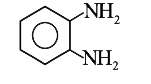
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
AMINES
VMC MODULES ENGLISH|Exercise IMPECCABLE|48 VideosAMINES
VMC MODULES ENGLISH|Exercise ILLUSTRATION|25 VideosAMINES
VMC MODULES ENGLISH|Exercise ENABLE|45 VideosALDEHYDES,KETONES & CARBOXYLIC ACIDS
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-M|10 VideosATOMIC STRUCTURE
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive )|67 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-AMINES-EFFICIENT
- A compound give violet flame rest and gives a white ppt with AgNO(3...
Text Solution
|
- In the above conversion m first step would be the reaction of ...
Text Solution
|
- Find out the major prodcut of the following reacton :
Text Solution
|
- The mojor product (70% to 80%) of the reaction between m-dinitrobenzen...
Text Solution
|
- The end product (z ) of the following reaction is :
Text Solution
|
- Which of the following will not react with CS(2)?
Text Solution
|
- In the given reaction
Text Solution
|
- which of the following reactions will not give a primary amine ?
Text Solution
|
- In the given sequence of reaction :
Text Solution
|
- The correct route for the above change is :
Text Solution
|
- The compound which gives H2O2 on treatment with dilute acid is
Text Solution
|
- Which of the following is more basic than aniline?
Text Solution
|
- The compound (B ) is :
Text Solution
|
- Consider the reaction given below :
Text Solution
|
- which among the following represents Y ?
Text Solution
|
- C(6)H(5)NH(2) + CS(2) underset(KOH (s))overset(Delta)rarr The end pr...
Text Solution
|
- The compound is subjected to following treatment stepwise (I...
Text Solution
|
- Complete the following reaction
Text Solution
|
- consider the following sequence of reaction : The product E...
Text Solution
|
- The strongest base among the following .
Text Solution
|