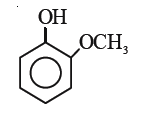
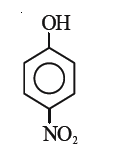
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALCOHOLS, PHENOLS & ETHERS
VMC MODULES ENGLISH|Exercise Efficient|98 VideosALCOHOLS, PHENOLS & ETHERS
VMC MODULES ENGLISH|Exercise Impeccable|100 VideosALCOHOLS, PHENOLS & ETHERS
VMC MODULES ENGLISH|Exercise Impeccable|100 VideosALCOHOLS, PHENOLS & ETHERS
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE - L|10 VideosALDEHYDES,KETONES & CARBOXYLIC ACIDS
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-M|10 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-ALCOHOLS, PHENOLS & ETHERS-Enable
- In the reaction CH(3)-overset(CH(3))overset(|)CH-CH(2)-O-CH(2)-CH(3)+H...
Text Solution
|
- The major organic product in the reaction . CH(3) - O- CH (CH(3))(...
Text Solution
|
- When HI reacts with diethyl ether in cold product would be :
Text Solution
|
- Which of the following pairs will not form ether?
Text Solution
|
- Phenol reacts with which of the following give bakelite ?
Text Solution
|
- Which one of the following compounds is most acidic :-
Text Solution
|
- C(6) H(5)NH(2) underset (0-5^(@))overset(HNO(2)//HCI)to X underset(H(2...
Text Solution
|
- The correct order of increasing acidic strength is .
Text Solution
|
- C(6)H(5) OH and C(6) H(5) COOH show difference in the reaction with
Text Solution
|
- Phenol is heated with phthalic anhydride in the presence of conc. H(2)...
Text Solution
|
- PhOH+CHCl(3) underset("Hydroxide")overset("Sodium")to product. The p...
Text Solution
|
- Phenol is more acidic than :
Text Solution
|
- Phenol gives . . . . . . . . Colour with neutral FeCl(3) solution :
Text Solution
|
- What is the product formed in the following reaction? C(6)H(5)OH + "...
Text Solution
|
- Asqirin is obtained by the reaction of salicylic acid with
Text Solution
|
- The product of the reaction of phenol with bromine water is :
Text Solution
|
- C(6)H(5)COCl+C(6)H(5)OH overset(NaOH)toC(6)H(5)COOC(6)H(5)+HCl This ...
Text Solution
|
- In the avove reaction the intermediate species are :
Text Solution
|
- How will you prepare benzene from
Text Solution
|
- Phenol is acidic , because :
Text Solution
|