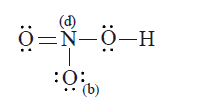Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING & MOLECULAR STRUCTURE
VMC MODULES ENGLISH|Exercise SOLVED EXAMPLES|20 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
VMC MODULES ENGLISH|Exercise PRACTICE EXERCISE|36 VideosCHEMICAL BONDING & CHEMICAL STRUCTURE
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|98 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL BONDING & MOLECULAR STRUCTURE -IN-CHAPTER EXERCISE-L
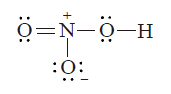
- Calculate formal charge (F) on nitrogen and Oxygen atoms marked 'a' an...
Text Solution
|
- In solid argon , the atoms are held together by
Text Solution
|
- Which of the following exhibits the weakest intermolecular forces?
Text Solution
|
- Among the following the weakest force of interaction is
Text Solution
|
- Covalent molecules are usually held in a crystal structure by
Text Solution
|
- Which of the noble gas has highest polarizability
Text Solution
|
- Dissoution of iodine crystals in carbon tetrachlorides solvent can be ...
Text Solution
|
- If NaCl dissolves in water then the nature of interaction between them...
Text Solution
|
- The attractive forces that exist between non polar molecules such as O...
Text Solution
|
- Which of these can explain the unusual contraction of water when heate...
Text Solution
|