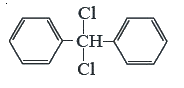
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HALOALKANES AND HALOARENES
VMC MODULES ENGLISH|Exercise PRACTICE EXERCISE - 1|6 VideosHALOALKANES AND HALOARENES
VMC MODULES ENGLISH|Exercise PRACTICE EXERCISE - 2|12 VideosHALOALKANES AND HALOARENES
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE - F|6 VideosHALOALKANES & HALOARENES
VMC MODULES ENGLISH|Exercise IMPECCABLE|100 VideosHYDROCARBONS
VMC MODULES ENGLISH|Exercise JEE Advanced Archive|121 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-HALOALKANES AND HALOARENES-SOLVED EXAMPLES
- Arrange the following in order of their Increasing basicity: H(2)O, ...
Text Solution
|
- Arrange the following in the order of their increasing reactivity in n...
Text Solution
|
- Write the structure of the major organic product expected from each of...
Text Solution
|
- Write the structure of the major organic product expected from each of...
Text Solution
|
- Identify the major product in the following reactions : C(6)H(5)-CH(...
Text Solution
|
- Aryl halides are less reactive than alkyl halides towards nuceophilic ...
Text Solution
|
- Optically active 2-bromobutane on treatment with NaI in acetone gives ...
Text Solution
|
- A compund X(C8H10O) upon treatment with alkaline solution of iodine gi...
Text Solution
|
- Predict the structure of the product in the following reaction :
Text Solution
|
- How will you prepare m-bromoiodobenzene from benzene (in not more t...
Text Solution
|
- Write the structures of the products : C(6)H(5)CH(2)CHClC(6)H(5) ove...
Text Solution
|
- Write the strucrtures of the products. (CH(3))(2)CH-OCH(3)underset("he...
Text Solution
|
- Identify A, B and C and give their structures
Text Solution
|
- The compound formed by reaction of 1, 1- dichloropropane with Zn can a...
Text Solution
|
- Which of the following compounds gives iodoform with I(2) + NaOH
Text Solution
|
- Identify Z in the following reaction sequence C(2)H(5)Ioverset(alc.K...
Text Solution
|
- The following compound is obtained when CH(3)CH(2)CHBr(2) is boiled wi...
Text Solution
|
- Which of the following statements is incorrect for ethylene dichloride...
Text Solution
|
- Which of the following structures correspond to the product expected, ...
Text Solution
|
- Chlorination of toluene in the presence of light and heat followed by ...
Text Solution
|