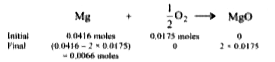A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- 1.0 g of magnesium is burnt with 0.56 g O2 in a closed vessel. Which r...
Text Solution
|
- 1.0 g of magnesium is burnt with 0.56 g O(2) in a closed vessel. Which...
Text Solution
|
- 1.0 g of magnesium is burnt with 0.56 g of O(2) in a closed vessel. Wh...
Text Solution
|
- 1.0 g of magnesium is burnt with 0.56 g O(2) in a closed vessel. Which...
Text Solution
|
- 1g of Mg is burnt with 0.28g of O(2) in a closed vessel. Which reactan...
Text Solution
|
- 1.0 ग्राम मैग्नीशियम को 0.56 ग्राम O(2) के बाद बन्द पात्र में जलाया गय...
Text Solution
|
- 1.0g of magnesium is burnt with 0.56 g O(2) is a closed vessel. Which ...
Text Solution
|
- 1.0 g of magnesium is burnt with 0.56 g O(2) in a closed vessel. Which...
Text Solution
|
- 1.0 g of magnesium is burnt with 0.56 g O(2) in a closed vessel. Which...
Text Solution
|