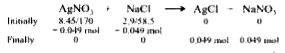A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- What is the mass of the precipitate formed when 50 mL of 16.9% solutio...
Text Solution
|
- What is the mass of the precipitate formed when 50 mL of 16.9% solutio...
Text Solution
|
- What is the mass of the precipitate formed when 50 mL of 16.9 % soluti...
Text Solution
|
- What is the mass of precipitate formed when 50mL of 16.9% solution of ...
Text Solution
|
- What is the mass of the precipitate formed when 50 mL of 16.9 % (w//v ...
Text Solution
|
- What is the mass of the precipitate formed when 50 mL of 17.0% solutio...
Text Solution
|
- What is the mass of the precipitate formed when 50 mL of 16.9% solutio...
Text Solution
|
- What is the mass of precipitate formed when 50 mL of 16.9% solution of...
Text Solution
|
- 50 ml 16.9% AgNO3 विलयन व 5.8% NaCl व विलयन के 50 ml को मिलाने पर प्रा...
Text Solution
|