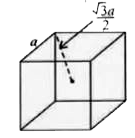
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MTG GUIDE-SOLID STATE -AIPMT/NEET MCQS
- AB crystallises in a body-centred cubic lattice with edge length 'a' e...
Text Solution
|
- A solid compound XY has NaCl structure. If the radius of the cation (X...
Text Solution
|
- A metal crystallises with a face-centred cubic lattice. The edge of th...
Text Solution
|
- The number of octahedral void(s) per atom present in a cubic close-pac...
Text Solution
|
- Structure of a mixed oxide is cubic close packed (ccp). The cubic unit...
Text Solution
|
- A metal has a foc lattice. The edge length of the unit cell is 404 pm....
Text Solution
|
- The number of carbon atoms per unit cell of diamond unit cell is
Text Solution
|
- If a is the length of the side of a cube, the distance between the bod...
Text Solution
|
- A given metal crystallises out with a cubic structure having edge leng...
Text Solution
|
- The vacant space in bcc lattice unit cell is
Text Solution
|
- The correct statement regarding defects in crystalline solids is
Text Solution
|
- Lithium has a bcc structure. Its density is 530 kg m and its atomic ma...
Text Solution
|
- The ionic radii of A^+ and B^- ions are 0.98 xx 10^(-10) m and 1.81 xx...
Text Solution
|
- In calcium fluoride, having the fluorite structure, the coordination n...
Text Solution
|
- Which is the incorrect statement?
Text Solution
|
- Iron exhibits bcc structure at room temperature Above 900 ^@C, it tran...
Text Solution
|
- A compound is formed by cation C and anion A. The anions form hexagona...
Text Solution
|