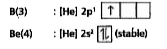Text Solution
Verified by Experts
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Long Answer Questions|24 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Very Short Answer Questions|85 VideosCHEMICAL EQUILIBRIUM AND ACIDS BASES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS |31 VideosENVIRONMENTAL CHEMISTRY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS|6 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES-Short Answer Questions
- What is isoelectronic series? Name a series that will be isoelectronic...
Text Solution
|
- Explain why cation is smaller and anion is larger in radii than their ...
Text Solution
|
- Arrange the second period elements in the increasing order of their fi...
Text Solution
|
- IE(1) of Na is less than that of Mg but IE(2) of Na is higher than tha...
Text Solution
|
- What are the various factors due to which the IE of the main group ...
Text Solution
|
- The first ionization enthalpy values (in kJ mol^(-1)) of group 13 elem...
Text Solution
|
- Would you expect the second electron gain enthalpy of oxygen as positi...
Text Solution
|
- What is the basic difference between the electron gain enthalpy and el...
Text Solution
|
- Would you expect IE(1) for two isotopes of the same element to be the ...
Text Solution
|
- Increasing order of reactivity among group - 1 elements is Li < Na < K...
Text Solution
|
- Assign the position of the element having outer electronic configurati...
Text Solution
|
- Assign the position of the element having outer electronic configurati...
Text Solution
|
- Predict the formulae of the stable binary compounds that would be form...
Text Solution
|
- Predict the formulae of the stable binary compounds that would be form...
Text Solution
|
- Predict the formulae of the stable binary compounds that would be form...
Text Solution
|
- Predict the formulae of the stable binary compounds that would be form...
Text Solution
|
- Predict the formulae of the stable binary compounds that would be form...
Text Solution
|
- Predict the formulae of the stable binary compounds that would be form...
Text Solution
|
- Write a note on the variation of metallic nature in a group and in a p...
Text Solution
|
- How does the covalent raduis increase in group 7?
Text Solution
|