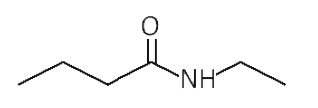
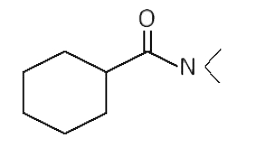
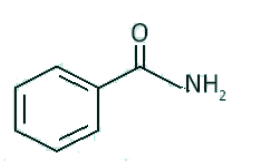
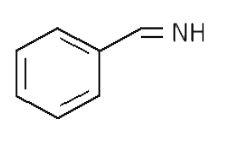
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The compound which can give Amine with KOH and Br(2) is-
Text Solution
|
- A compound A has a molecular formula C(7)H(7)NO . On treatment with Br...
Text Solution
|
- निम्नलिखित में से कौन सा नाइट्रोजन युक्त यौतगिक हॉफमैन ब्रोमामाइड अभिक...
Text Solution
|
- A compound A has a molecules formula C(7)H(7)NO . On tratement with Br...
Text Solution
|
- किसी ऐमाइड की Br(2) तथा KOH के साथ क्रिया कराकर प्राथमिक ऐमीन प्राप्त ...
Text Solution
|
- Which one of the following compounds when heated with KOH and a primar...
Text Solution
|
- Which one of the following compounds when heated with KOH and a primar...
Text Solution
|
- Give the IUPAC name and structure of the amine obtained when 3-chlorob...
Text Solution
|
- Compound A is the following reaction is A overset(NH(3)//Delta)to ...
Text Solution
|