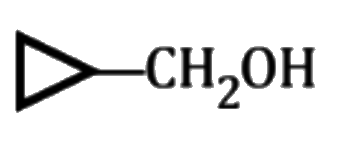
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following alcohols do not give white turbidity on treatme...
Text Solution
|
- Which of the following alcohol gives the white turbidity imediately wi...
Text Solution
|
- Which alcohol is most reactive towards HCl in the presence of anhydrou...
Text Solution
|
- Alcohols which can give white turbidity immediately on reaction with L...
Text Solution
|
- Which of the following alcohols do not give white turbidity on treatme...
Text Solution
|
- STATEMENT - 1 : gives turbidity with ZnCl(2)//HCl in 5 minutes. S...
Text Solution
|
- Lucas test is usedto make distinction between1^(@) 2^(@)"and"3^(@)alco...
Text Solution
|
- Neopentyl alcohol on treatment with HCl and anhydrous ZnCl(2) gives
Text Solution
|
- The alcohol that produces turbidity immediately with ZnCl(2)/conc. HCl...
Text Solution
|