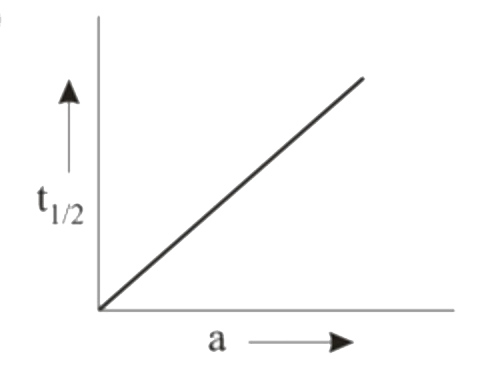
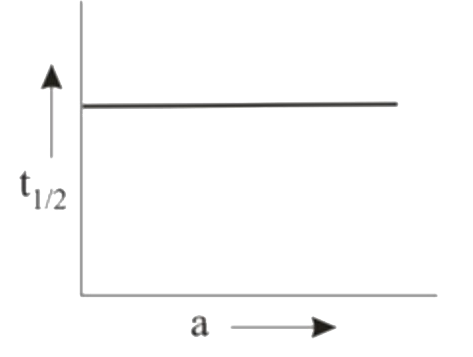
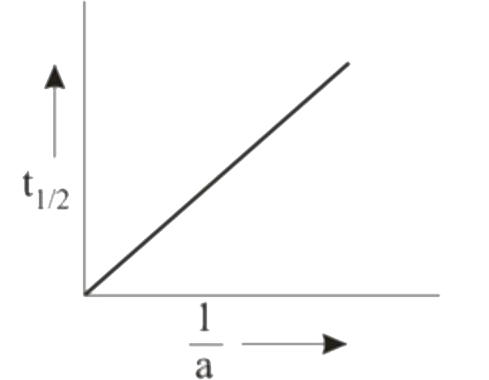
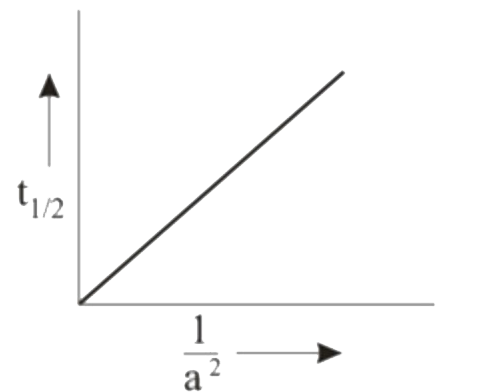
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following is correct representation of the variation of h...
Text Solution
|
- For a zero order reaction, the half-life periof is independent of the ...
Text Solution
|
- A zero-order reaction, Ararr Product, with an initial concentration [A...
Text Solution
|
- सिद्ध कीजिए कि शून्य कोटि कि अभिक्रिया का अर्द्ध-आयुकाल अभिकारक की प्र...
Text Solution
|
- Half life in a first order and zero order reaction are same then ratio...
Text Solution
|
- What is half life period of a reaction ? Show that half period for a z...
Text Solution
|
- Which of the following is correct representation of the variation of h...
Text Solution
|
- Half life period of zero order reaction is ........... proportional to...
Text Solution
|
- In the initial cocnentration is reduced to 1/4th of the initial value ...
Text Solution
|