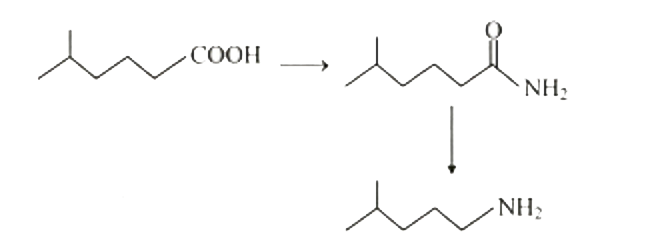
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the following conversion : Amongst the following, how ma...
Text Solution
|
- Write short notes on the following : i. Carbylamine reaction ii. Diazo...
Text Solution
|
- Mendius reaction involves the reduction of-
Text Solution
|
- Which of the following conversion can be carried out by Clemmensen red...
Text Solution
|
- Write the chemical equations involved in the following reactions: (i) ...
Text Solution
|
- Write chemical reaction each to illustrate the following : (i) Hofmann...
Text Solution
|
- Consider the following conversion : Amongst the following, how ma...
Text Solution
|
- Write short on the following : (i) Carbylamine reaction (ii) Hofma...
Text Solution
|
- GABRIEL PHTHALIMIDE SYNTHESIS
Text Solution
|